This post was earlier cross-posted at Leonid Schneider's site, hence the unfrivolous tone. The version there is improved by Leonid's editing and frame-story.
Here is a foretaste of what is to come, to whet your appetite during a lengthy exposition.This post returns to the theme of illustrations in the biomedical literature. Specifically, to the theme of electrophoresis gels -- the raw material of research, several stages removed from the numbers that are eventually reported as data. I am far from the first to observe that the point of using them to illustrate a scientific paper is really to show that the experiments were conducted as claimed… much as if every paper on the Higgs boson had to be illustrated with photographs of CERN operators drinking coffee and monitoring dials in the Large Hadron Collider control room.
So a choice of the wrong images is disconnected from the reliability of the data. It is understandable, then, if researchers enhance their gels for publication (or assemble them in software), if that's what the editors demand. Yet editors are not well-pleased when the use of Appearance-Enhancing Photoshop comes to light, for it speaks of a dismissive attitude and a disturbing lack of pedantry on the part of the authors.
----------------------------------------------------------------
A long series of science-fiction thrillers teaches us that the name
'Andromeda' has bad associations; things always end badly. But the
lesson was lost on the people who created Andromeda Biotech Ltd. to
commercialise a synthetic polypeptide, DiaPep277, on behalf of the Weizmann Institute.

I am loath to spoil the suspense, but I should say here at the start that
DiaPep277 proved in the end to have no clinical value in slowing the progression of Type-1 Diabetes (T1D). And there is no shame in that, for most promising new drugs and interventions leave their efficacy behind them in the laboratory when they venture out into clinical trials. This ensures that tabloid journalists have a constant supply of 'New Wonder Cure for X' to write about, and provides pharmaceutical companies with an excuse to charge 10-fold when one of their new drugs is (initially) sellable.So T1D is an autoimmune disease, caused when a hyper-sensitive immune system targets the insulin-producing ß-cells of the pancreas for detruction. The idea was that the specific antigen they over-react to is HSP-60 -- one of the family of Heat Shock Proteins (which is totally the name of my new Cramps tribute band). HSP-60 is ubiquitous in human tissues, so it was not clear why an irritable immune system should take special umbrage to its presence in the pancreas; nor was it obvious why stimulating the immune response, with injections of the synthetic analog DiaPep, should reduce the damage it wreaks.
This leads us to Irun Cohen of the Department of Immunology of the Weizmann Institute, and his radical maverick Immunological Homunculus paradigm. The details of this escape me, but other laboratories have been slow to take it up, rather like Cap'n Redbeard Rum and his radical views on maritime crew levels. Readers may be familiar with the Weizmann Institute.
Now Professor Cohen is the first to admit that his 'autoimmune vaccination' concept is rather out there, but he is all about the Conceptual Paradigm Shift, and the Big-Picture Perspective, and the Interdisciplinary Out-of-the-Box Thinking. Also the Bold Metaphors.
"How did we arrive at the concept of vaccination therapy and how did we suspect that peptide p277 might turn out to be an effective vaccine. How does one dare go from mice to people?"So far he has not been driven to confront the scientific establishment from the turret of a castle laboratory, brandishing a fist at the storm-clouds and shouting "You hidebound fools! You mocked my theories and robbed me of my Nobel! But I'll show you all!!" Let us hope that it does not progress to that.
"The idea of vaccination for autoimmune disease was quite foreign to the thinking current at the time among autoimmunologists; the vaccination concept reflected my initial training in infectious disease".
In company with his PhD student and protegée Dana Elias, Cohen developed his insight into the "HSP-autoimmunity-causes-T1D" theory, and from there to this 277 polypeptide -- tailored to contain the antigenic flags of HSP-60, but boiled down to only 24 amino acids for easy production from vats of recombinant E. coli. There was a 1991 mouse-model paper in PNAS and a 1994 letter to Lancet. Dr Elias was one of six scientists employed by Andromeda Biotech who declined to comment on or cooperate with the retraction of DiaPep-clinical-trial papers in late 2014.
But I have got ahead of myself there. The company Peptor had been spun off from the Weizmann in 1993, vested with the intellectual-property rights to DiaPep277 and tasked with bringing it to market. It sold the rights to Avantis in 2002 but re-acquired them in 2003. In 2004 Peptor begat DeveloGen, which in 2007 begat Andromeda Biotech.
Initial-stage trials from several groups (2007-2009) agreed that DiaPep277 had no serious side-effects on recipients, and did provoke responses of some kind from immune systems, although any remission in the progress of T1D was difficult to discern (prompting efforts to broaden the scope of potential patients, and simultaneously to narrow it in search of some subgroup who would benefit). There was enough evidence to begin large-scale multi-centre trials, with distinguished diabetologist Itamar Raz recruited by now to oversee the project. And there was gloating from the Weizmann over the vindication and the discomfiture of the skeptics and nay-sayers.
Meanwhile a group at the Department of Immunology had been tasked with finding (a) evidence to support the rickety rationale for vaccinating against diabetes, and (b) specific cellular mechanisms through which DiaPep277 might work the immunomodulatory magic expected from it, and a series of papers emerged. The artistic quality of the illustrations for these papers brought them to the attention of the anonymous whistle-blowers, data-integrity extremists and blackboard monitors who frequent PubPeer. Now, with the backstory out of the way, I will plagiarise their contributions.
----------------------------------------------------------------
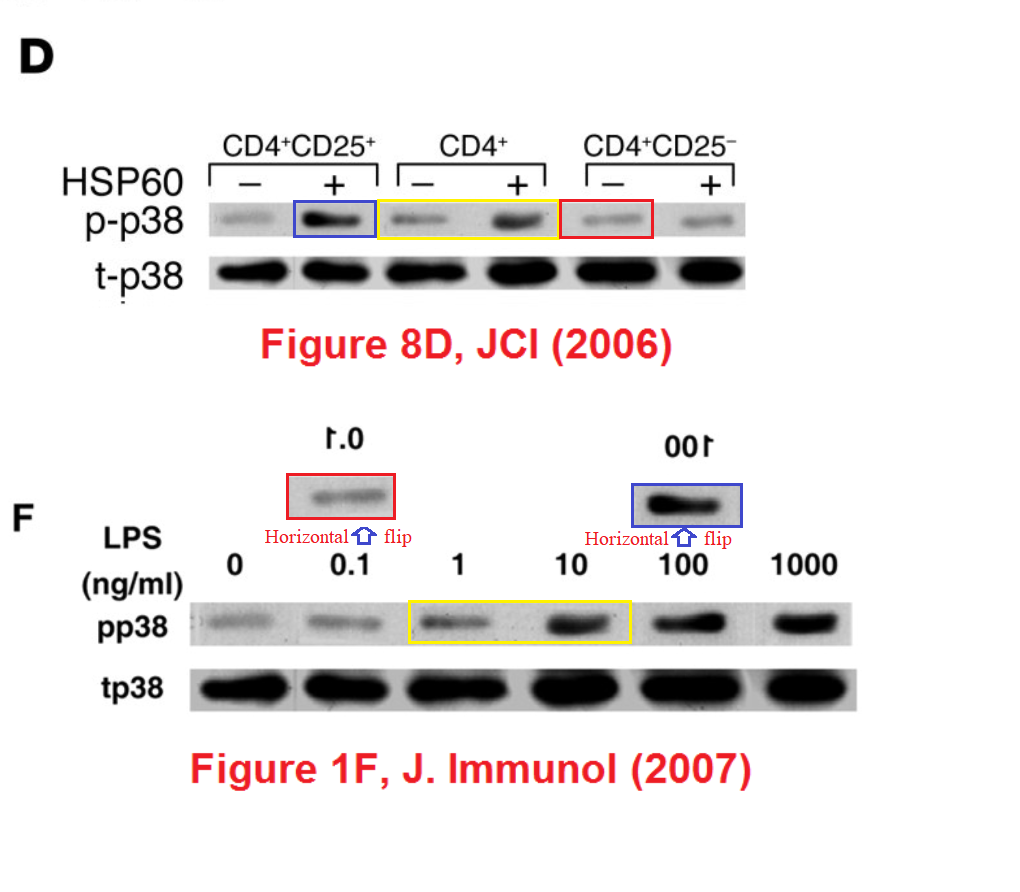
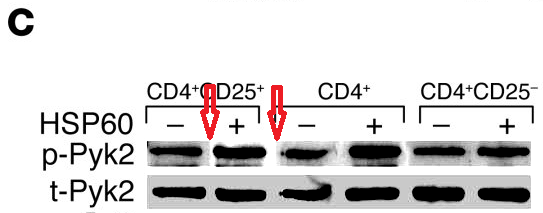
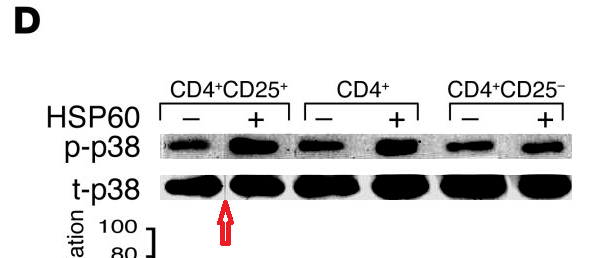
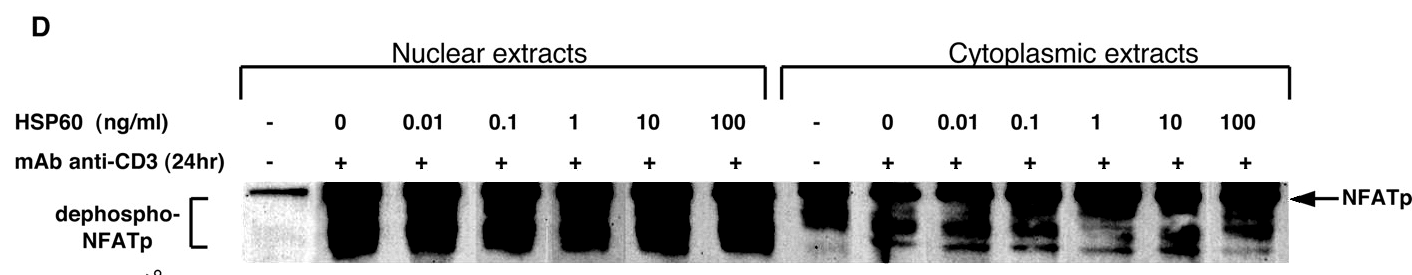
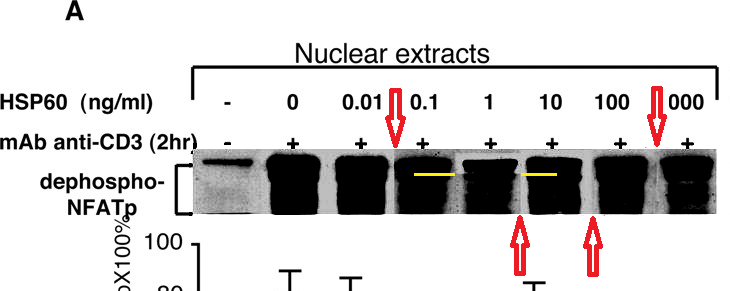
Perhaps we should begin with "Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling" (Zanin-Zhorov et al., J. Clinical Investigation, 2006), for it became the subject of press releases from the Weizmann to showcase the team (admitting in passing that the development of DiaPep had hitherto been driven by intuition and aspirational metaphors). Contrast adjustments to Figures 8C and 8D expose changes in the background, delineating the second and first lanes of the p-Pyk2 and t-p38 bands respectively: suggesting that these lanes were not originally part of the same experimental session as their neighbours, but were spliced in digitally.
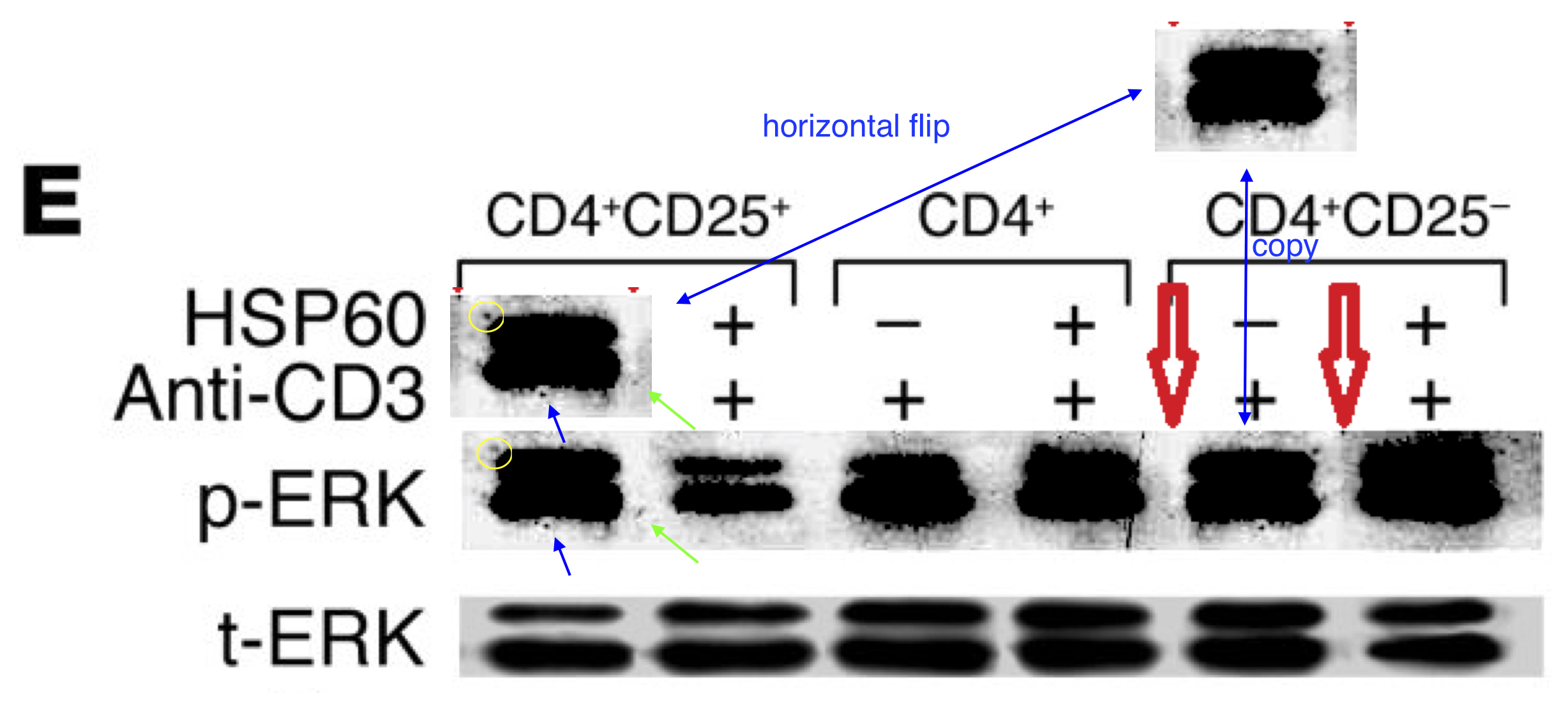
These bands show the amounts of a particular protein in its phosphorylated form, and also unphosphorylated, so that the former can be expressed in relative terms, corrected for experimental conditions; while lanes distinguish conditions: three types of T cell, with and without HSP-60.
In itself this is no great deal, but splice lines also delineate the 5th lane of the p-ERK lane in Figure 8E, purportedly from CD4+CD25- cells. Except it is a copy of the first lane (CD4+CD25+ cells), flipped horizontally to reduce its self-similarity. The point of the exercise had been to demonstrate the equivalence of these two cell types in the absence of HSP-60, and one has to conclude that the actual evidence was not strong enough.
----------------------------------------------------------------
Or perhaps we should begin in media res with "Heat Shock Protein 60 activates cytokine-associated..." (Zanin-Zhorov et al., J. Immunology, 2007), for it is a tour-de-force, on which the entire panoply of cosmetological enhancements has been deployed. The construction of the illustrative bands involved the duplication of numerous proteins, variously transformed.With their duplicate lanes only horizontally flipped, Figures 5F and 5H seem anodyne.
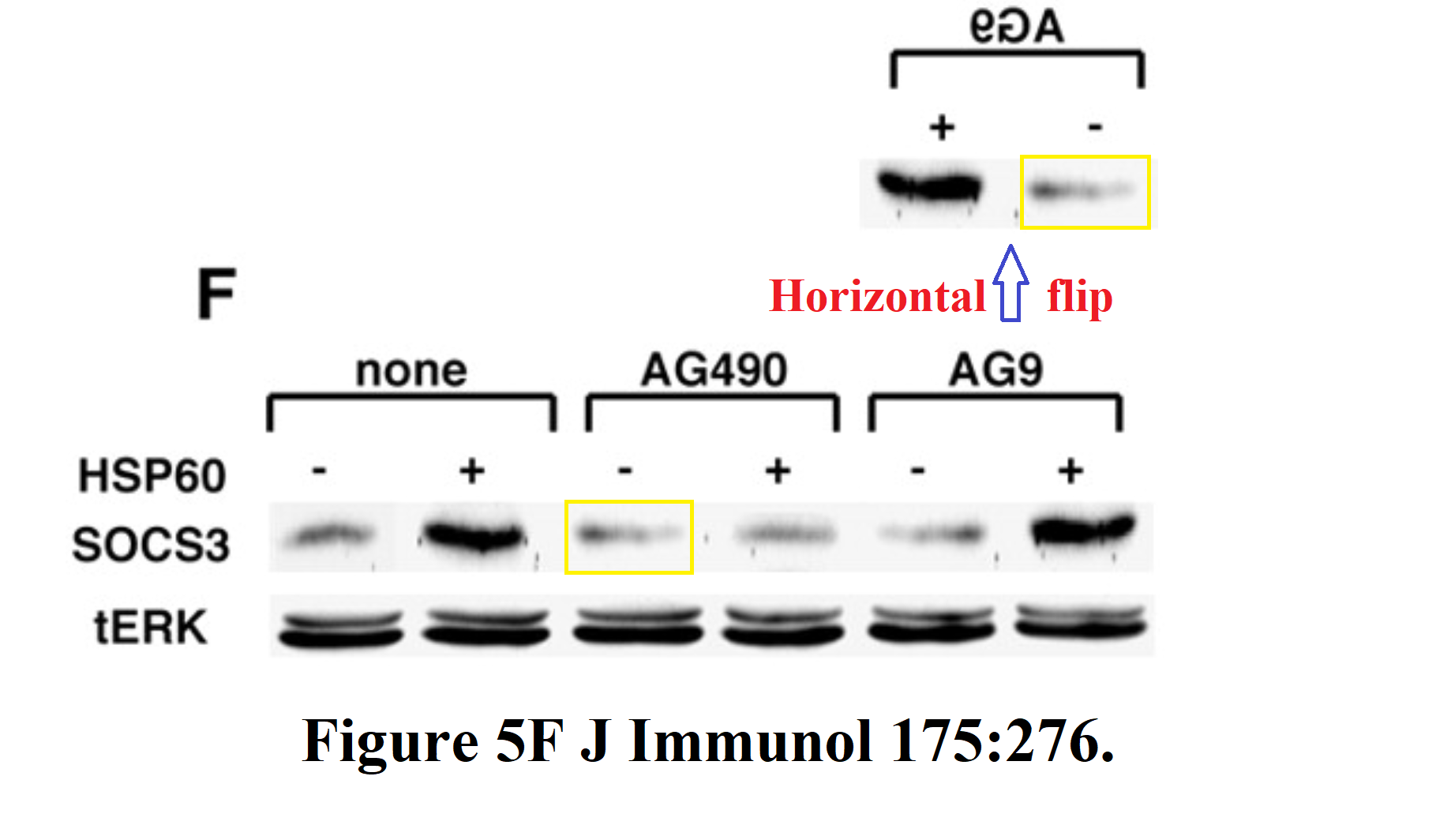

Figure 6A reveals another horizontally-flipped copy in its SOCS3 band, while in 6D, the overall darkness of the 6th lane of the SOCS3 band has been increased by a selective over-exposure, as is revealed by adjusting the contrast for that panel.

Figure 6E has 13 lanes, from T-cell cultures exposed to different levels of p277 or p30 ... or only 10 lanes, if one discounts the
In Figures 3E and 3F, as well as the replication of lanes within their respective reference bands tPyk2 and tAKT, the sixth lane of tAKT appears to be a copy of the corresponding pAKT lane... over-exposed, and with a crude attempt to erase the supernumerary streak just beneath it (characteristic of pAKT).
A whole three-lane neighbourhood was rotated 180° to construct the tMLC reference band in Figure 4B, while 4A can boast a duplicated lane in the pMLC band and a slightly-rotated duplicate tMLC lane.
----------------------------------------------------------------
The same panoply is on show in "Heat shock protein 60 inhibits Th1-mediated hepatitis model..." (Zanin-Shorov et al., J of Immunology, 2005). Attention first turned to splices around the second lane in the T-bet band of Figure 8A, which appears "looks slotted into panel" in the manner of intarsia; then on the fact that three of the other lanes are identical (though mirrored in one appearance).
Further showcases of extensive lane re-use are Figures 6A and 6E:


Figures 6B and 6D display only a single flipped lane each to make up their full complement:

Figures 7A, 7B, 7C provide some visual variety. Here the point of each lane is the amount of protein expressed in the top streak, relative to the total protein in all the other streaks, so the streaks are not widely resolved and the lanes resemble a collection of flowerpots. On close inspection, some of these flowerpots appear twice or even thrice within and between the separate panels (reflected and sometimes at longer exposures), representing different conditions -- sometimes purported as extracts of cell nuclei and sometimes of cytoplasm.
So Figure 7 was necessarily assembled like a stamp collection, in Photoshop rather than in the laboratory, and it was no surprise that adjusting the contrast would reveal vertical splices around separate flowerpots.
It was a surprise that one lane would have horizontal splice lines, indicative of a top streak that had been underexposed relative to the rest of the lane (lowering the ratio of protein content).
----------------------------------------------------------------
"T cells respond to heat shock protein 60 via TLR2" (Zanin-Shorov et al., FASEB, 2003) is less of a rich confection. In Figure 2A, the PBMC lane in the TLR-4 band was compressed vertically to 50% to produce the corresponding horizontal streak in the TLR-2 band, although its insertion there left a wide splice.
----------------------------------------------------------------
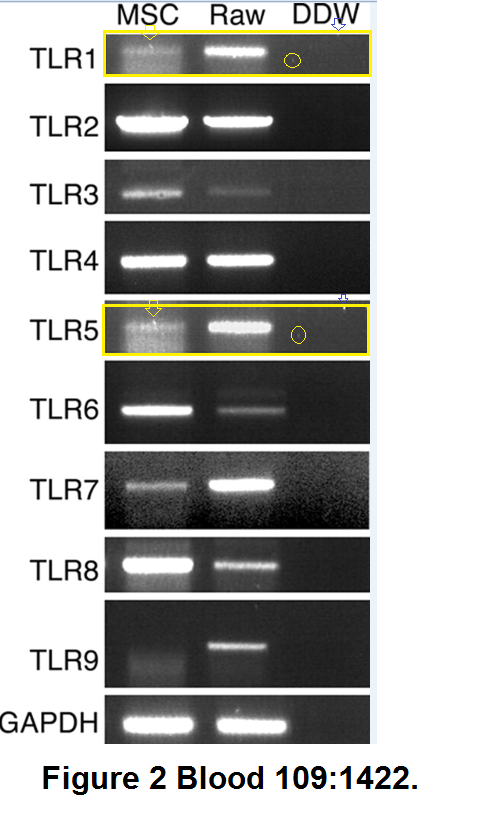 "Toll-like receptors and their ligands control mesenchymal stem cell functions" (Pevsner-Fischer et al., Blood, 2007), "Fibronectin-associated Fas ligands... " (Zanin-Zhorov et al, J. Immunology, 2003), and "Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling" (Zanin-Zhorov et al., J Immunology, 2007) were not directly related to the vindication of the DiaPep277 vaccination, but we include them anyway for the sake of the largely-overlapping co-authorship and methods.
"Toll-like receptors and their ligands control mesenchymal stem cell functions" (Pevsner-Fischer et al., Blood, 2007), "Fibronectin-associated Fas ligands... " (Zanin-Zhorov et al, J. Immunology, 2003), and "Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling" (Zanin-Zhorov et al., J Immunology, 2007) were not directly related to the vindication of the DiaPep277 vaccination, but we include them anyway for the sake of the largely-overlapping co-authorship and methods.The first is illustrated with RNA-PCA reactions rather than electrophoresis results for proteins, but the beautification tenchique is familiar: the vertical expansion of a copied gel to reduce its self-similarity.
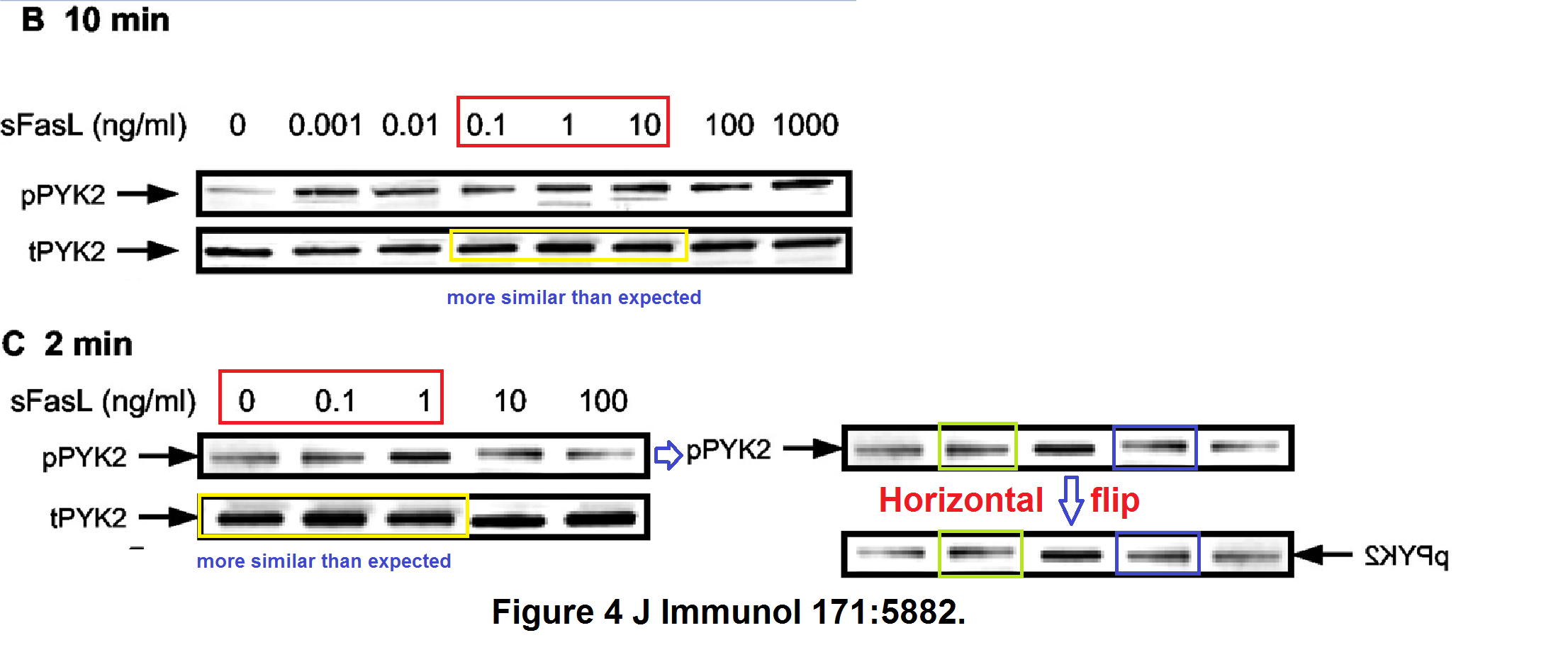
In the second, the pPyk2 and tPyk2 band are reused between Figures 4B and 4C, while two of the pPyK2 lanes within 4C are mirror-image copies.
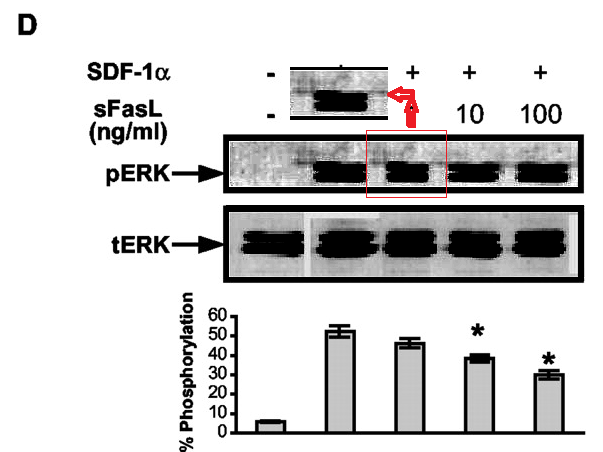
Bear in mind that lanes in the tERK band of Figure 5D are loading controls, to normalise the corresponding lanes in the pERK band. So it is cause for concern when we adjust the contrast on that panel and find that the second pERK lane is simply a copy of its neighbour (horizontally stretched), while the corresponding tERK lane has a surrounding moat of splices, as if the band had been assembled from multiple sources.
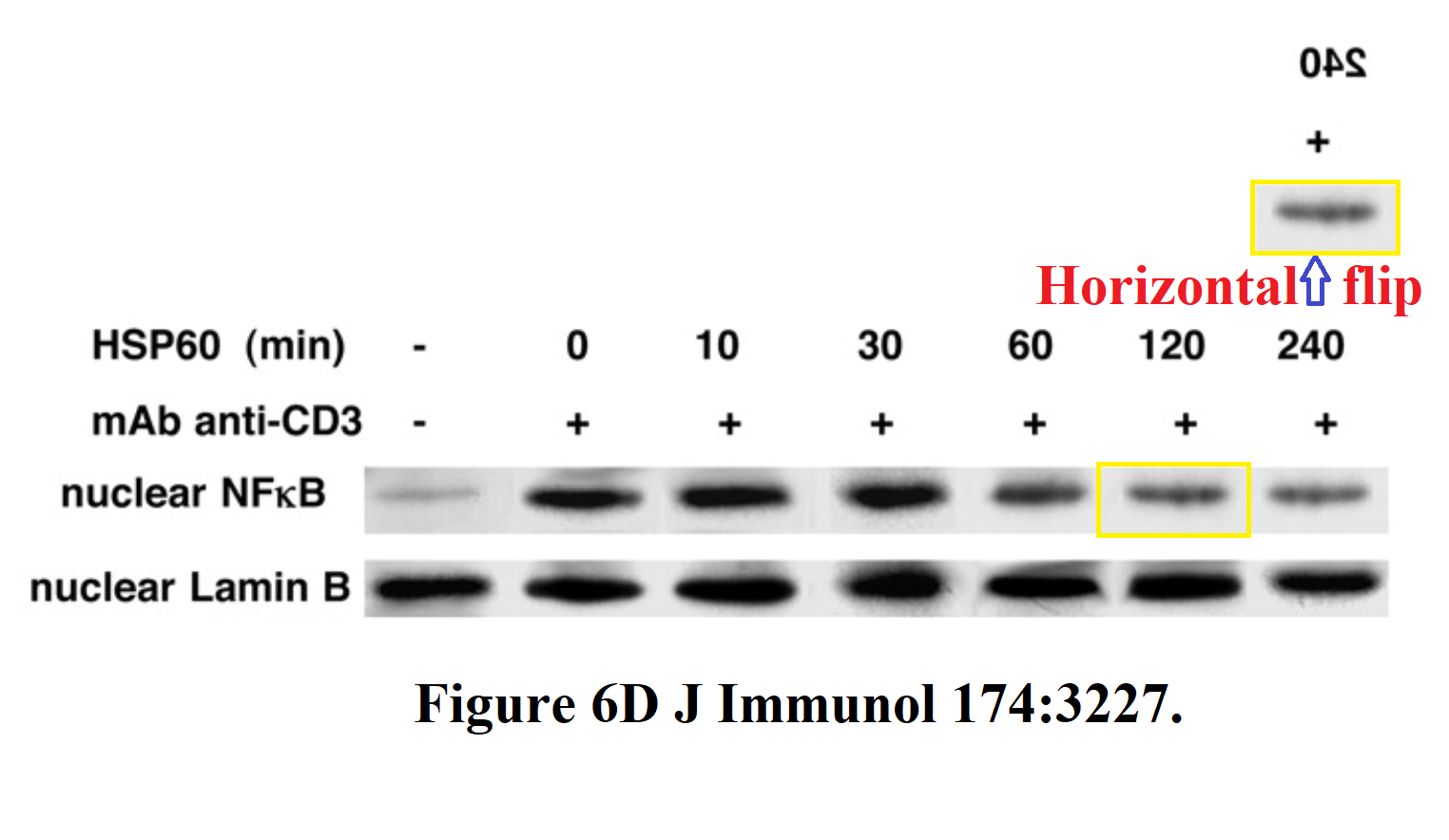
In the third of these out-takes ("Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling"), the 3rd and 6th lanes of the pPyk2 band in Figure 1E are the same, apart from a horizontal flip.
In Figure 1F, the pp38 band shows the phosphorylation of a protein in response to six levels of lipopolysaccharide, relative to the background levels of protein in the tp38 band. Rather alarmingly, the same tp38 control band had previously appeared in 2006 (where we started), showing the background in three varieties of T-cell, with or without HSP-60. Indeed, lanes 1, 3 and 4 of the pp38 band had also appeared in the same context, but with a different lane 6, while lanes 2 and 5 swapped places and flipped horizontally.
That doesn't happen by accident.
----------------------------------------------------------------
Two or three other papers from the team targetted the question of DiaPep277's mechanism, but they were not illustrated with gel images and have escaped the cold unsympathetic scrutiny of the PubPeer contributors.
----------------------------------------------------------------
Finally, Zanin-Zhorov and Cohen reviewed this work in Frontiers in Immunology (2013), providing an entry into their oeuvre by way of the References list. Of interest here is the authors' declaration that they had no commercial relationship that could be contrued as a Conflict of Interest (despite Cohen's position as Andromeda's "Clinical Advisory Board Member"). It may be that the term "COI" is understood differently in the Frontiers universe, but in 2014, in the Clinical-trial report, Cohen did feel that board membership was worth citing.But again I am getting ahead of myself for mentioning the 2014 Diabetes Care paper, which was retracted the same year.
Briefly: As the DIA-AID trial drew to a close, preliminary results were positive for one clinical end-point, but this posed the puzzle that a second clinical criterion showed no difference between DiaPep277 and placebo... it was as if someone had gone through the data, improving results for the first criterion by finding excuses to exclude unresponsive patients. And this was indeed what had happened. And there were retractions, and gnashing of teeth, and the new owners of Andromeda Biotech were not well-pleased, for on the basis of those preliminary results they had paid well for the company, thinking that they were acquiring a pharmaceutical goldmine.
Of course those clinical-trial shenanigans are not directly linked to the image cosmetology in the Weizmann Department of Immunology. But both could be symptomatic of a wider systemic problem -- a shared attitude that if results of an experiment are not sufficiently convincing, but you have a strong enough feeling about what the results should have looked like, then it is fine to improve them with the appropriate erasure of defects.
Let us hope the whole story doesn't inspire a sequel.













1 comment:
This is what happens when you attempt to execute a paradigm shift but don't know how to use a clutch.
Post a Comment