This post was earlier cross-posted at Leonid Schneider's site, hence the nonfrivolity and Explaining Voice. The version there is improved by Leonid's editing and frame-story.
The comments for that version include link to (A) fakes from X.-K. Wang's other students which were left on the cutting-room floor, (B) a Chinese translation with Figures improved by explanatory arrows and annotations (thx. anonymous Chinese translators!), and (C) a University investigation.
TigerBB8 reports more about links among the present Dramatis Personae.
The Brocken Spectre is a phenomenon seen from a mountain peak, looking down on clouds or fog with the sun behind one. "Brokenspectra" is a term I made up just now for unnatural wavelength functions - X-ray or IR or Raman spectra (and including X-ray diffraction patterns as honorary members of the club) - stitched together from spare parts, preferably in a laboratory in a Carpathian castle while angry peasants gather at the gates with pitchforks and torches.
Here is one to whet your appetite. I think it is some kind of mutant bar-code containing a hidden message.
 |
| Li et al. 2017 |
 |
| Jin, Shen & Sun, 2014 |
Leonid's readers will be disappointed by the absence of manipulated Western Blots from today's post. Instead there are electron-microscopy images of a protean nature, shifting their identities on every appearance; and Brokenspectra, including the first sightings from the high-end extreme of EXAFS (Extended X-Ray Absorption Fine Structure), which may serve as some consolation. Also versatile scholars in the Renaissance mould of Aldrovandi or Athanasius Kircher, breaking out from the narrow confines of their nominal specialty to publish in physical chemistry; and there are unusual patterns of citation. The central characters are Yubing Sun of the Chinese Academy of Sciences, a prolific and well-funded researcher at Hefei Institute of Physical Sciences (Institute of Plasma Physics) and North China Electrical Power University (NCEPU); and his frequent co-author, Xiangke Wang - Sun's boss at NCEPU.
But we should begin at the end: with
[1]. Enhanced adsorption of Zn(II) onto graphene oxides investigated using batch and modeling techniques (2018).
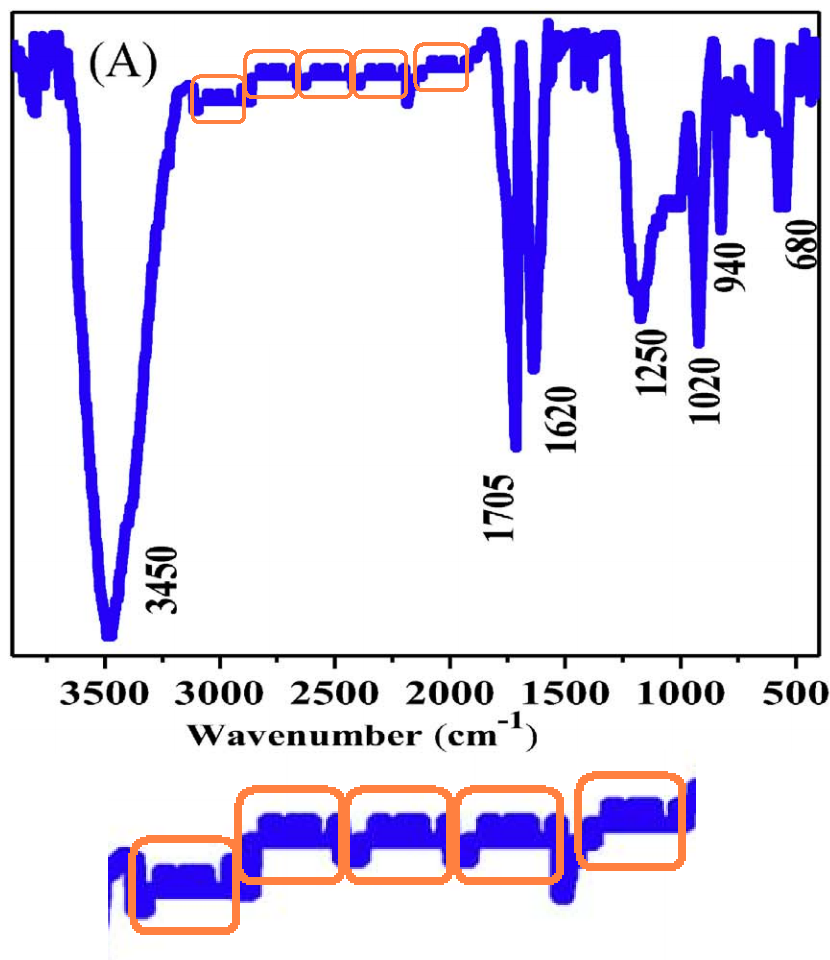
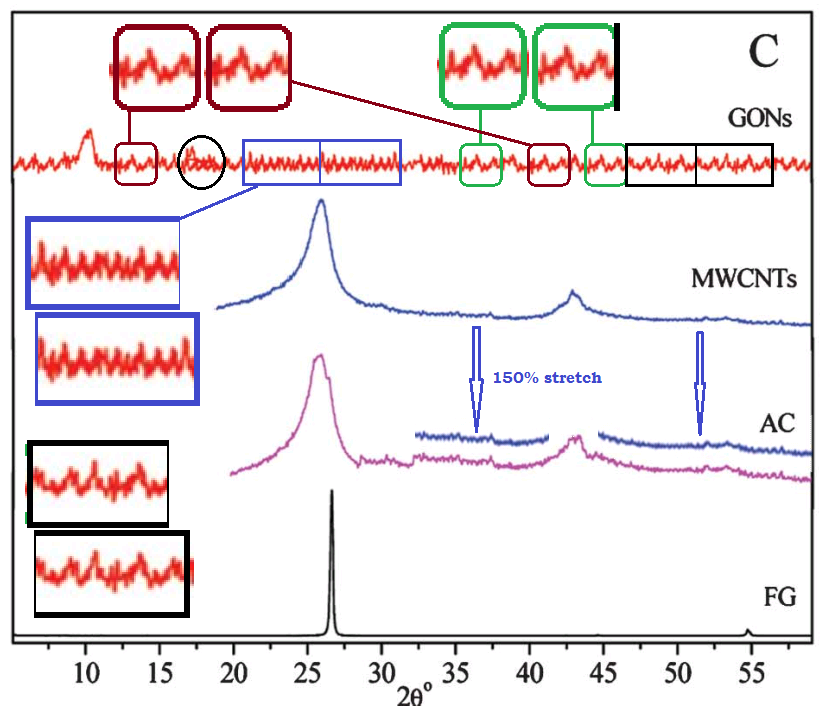
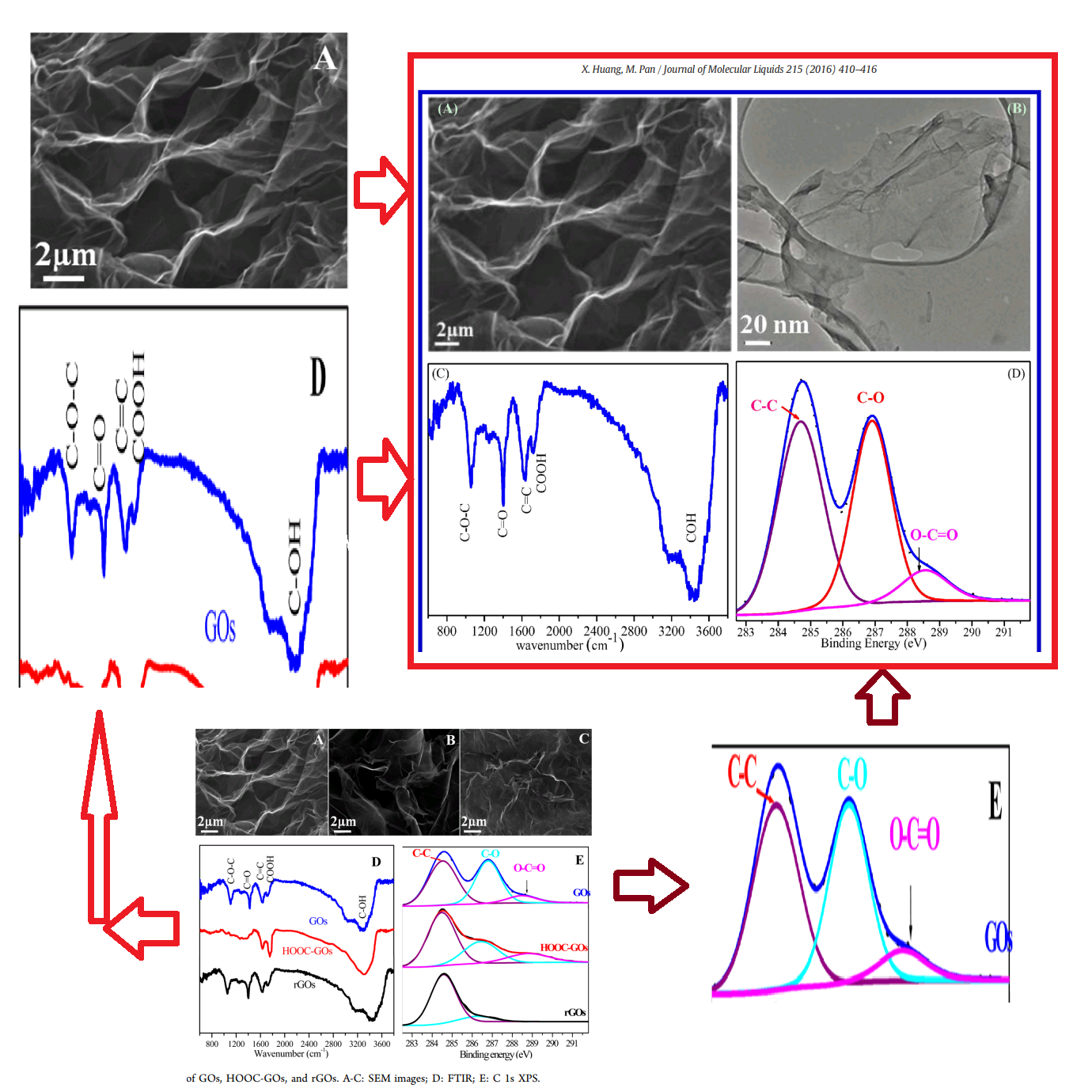
This excursion into advanced materials science is atypical for the lead author Min Pan, of Xiamen University of Technology, whose career centres on the treatment of sewage and slaughterhouse wastewater. The paper raises some concerns, as featured in a PubPeer thread. In particular, Figure 1 sets out to characterise the Graphene Oxide (GO) in various ways. But stuttering in the XRD contained in panel 1(E) hints at some heinous malfunction in the diffraction equipment.
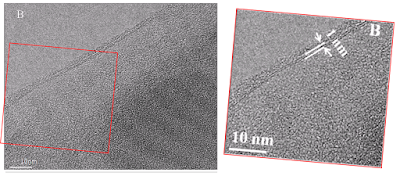
Figure 1(B) is a TEM (Transmission Electron Microscope) image of the experimental GO, but a section of it had previously been enlarged and slightly rotated [at right] to illustrate GO3, in [2]:
[2]. Tuning the chemistry of graphene oxides by sonochemical approach: Application on adsorption properties (Yubing Sun et al., 2015).
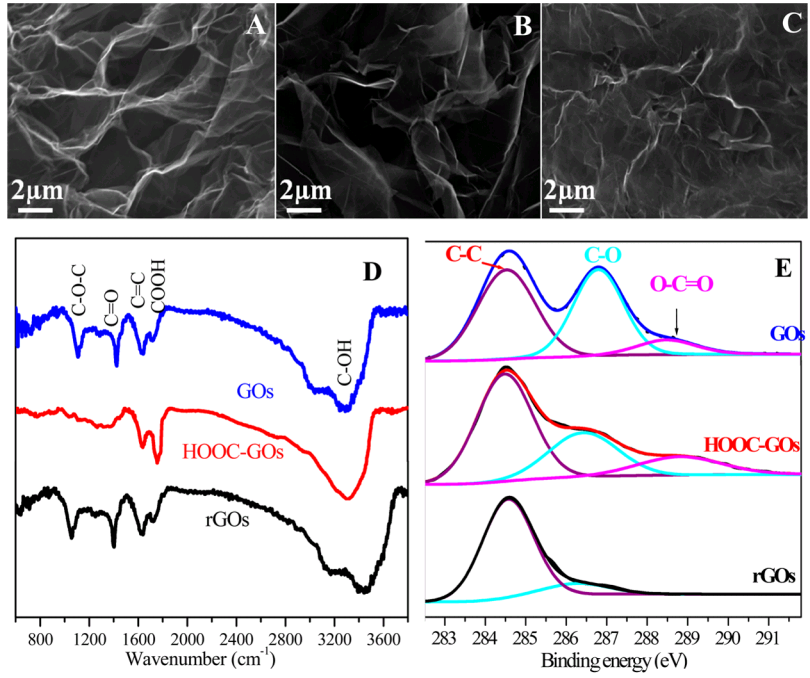
The SEM (Scanning Electron Microscope) image of the experimental GO had previously illustrated "HOOC-GOs" when it appeared in Fig 1(B) [right] of [3]:
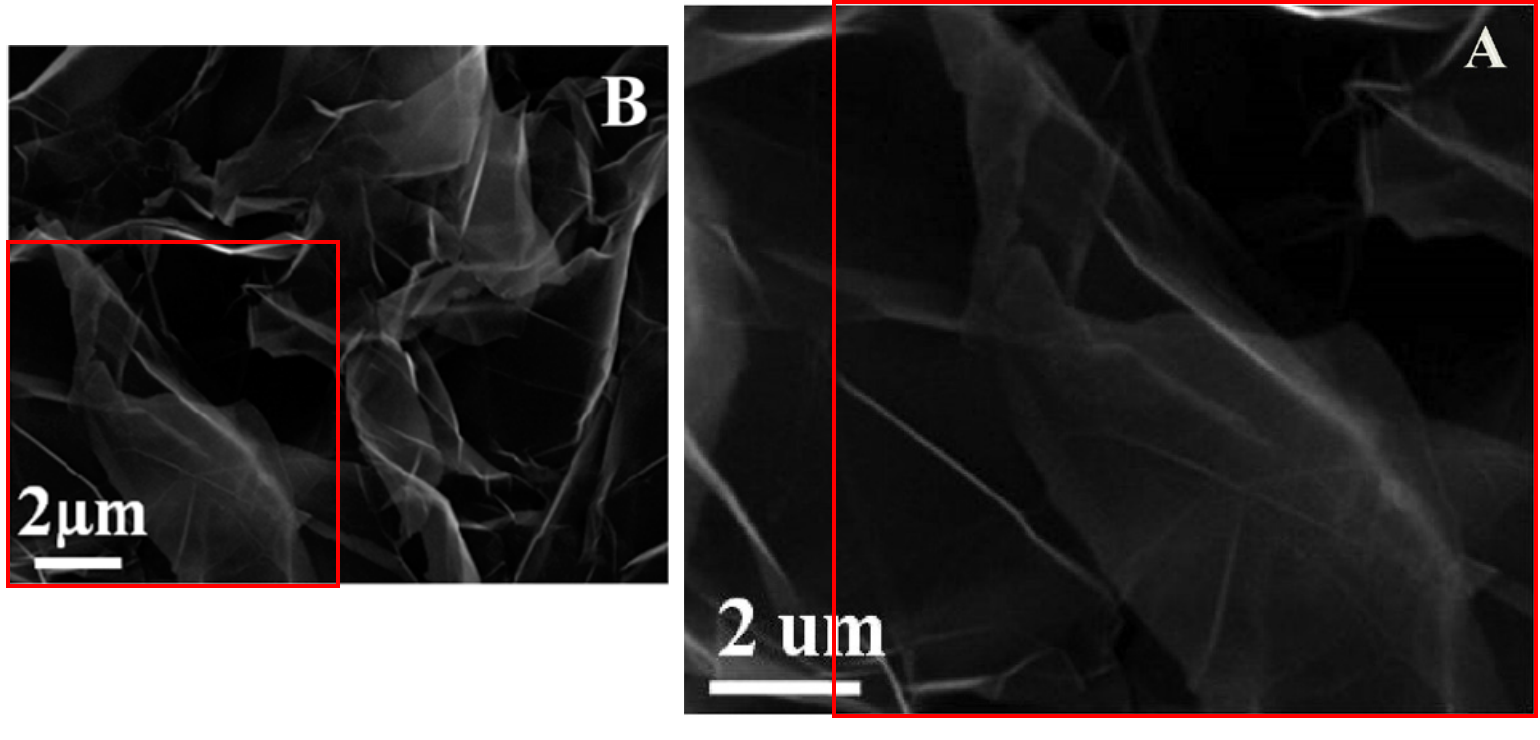
In fact Fig 1 of [3] has undergone some remarkable migrations. For convenient referral I show it over at the right. As noted, Panel 1(B) is a SEM of "HOOC-GOs". Except when it illustrates "sulfonated graphene oxide" (GO-OSO3H):
Note that one image is not simply an enlarged detail of the other: they overlap, each with its own unique segment, suggesting that two sets of authors cut down a single larger Ur-version, in different ways. Note also that this is now a different paper: we have set Min Pan's oeuvre aside for the moment, and turned to Ting Yao, of the Air Force Logistics College in Xuzhou. Yao has published on aspects of aviation oil and remediation of contaminated environments. But at some point she gained access to a supply of rare-earth elements and wrote [4]:
[4]. Adsorption of Eu(III) on sulfonated graphene oxide: Combined macroscopic and modeling techniques (Ting Yao et al, 2016).
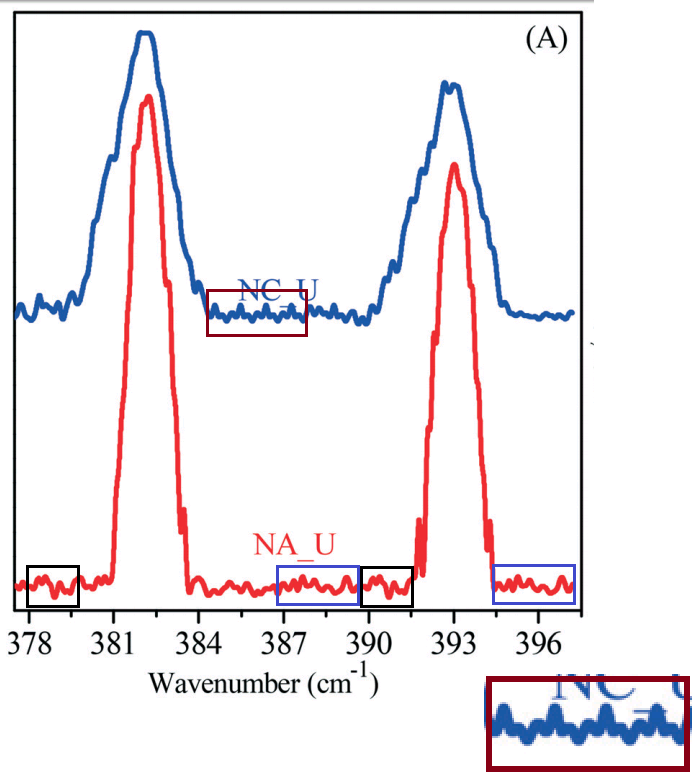
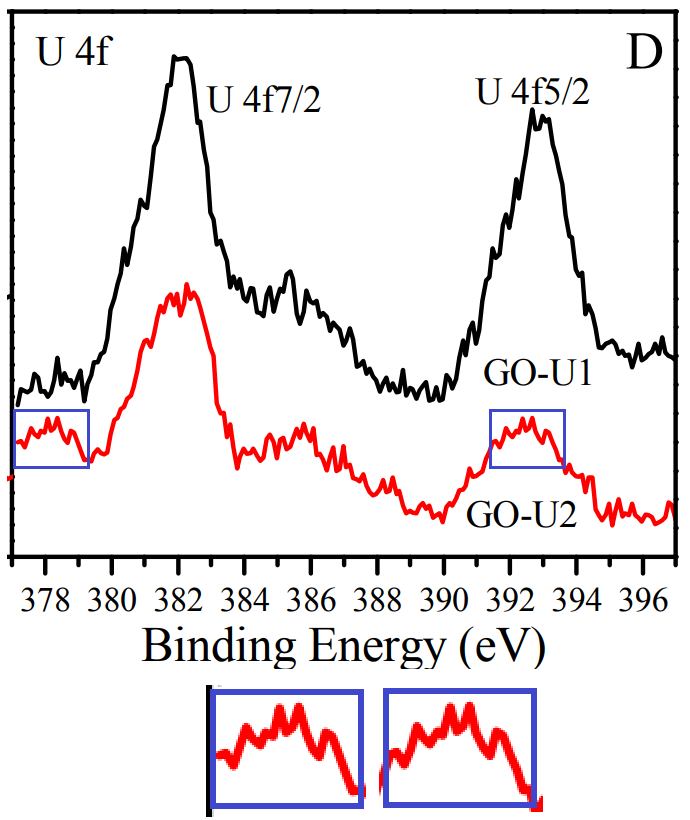
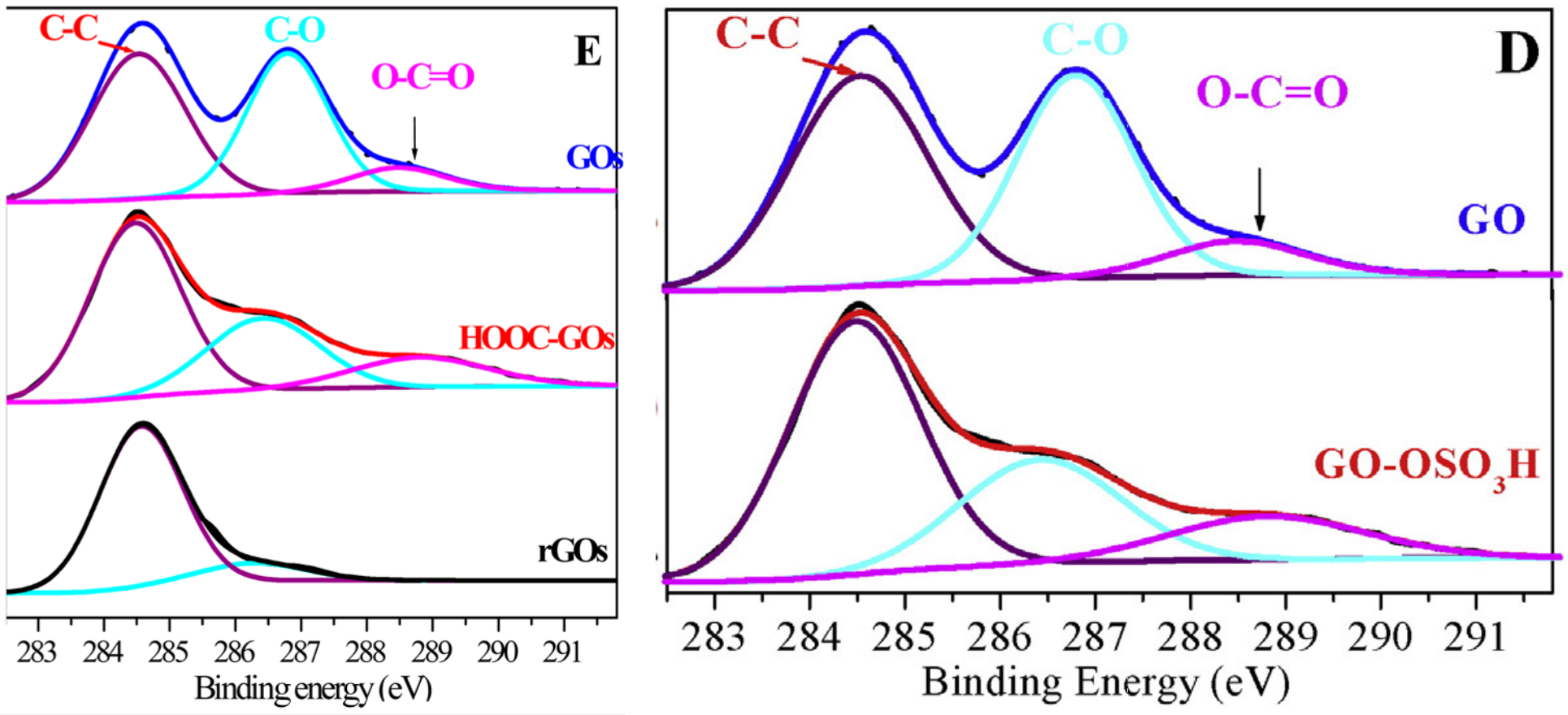
Figure 1 of [4] characterises the GO-OSO3H in several ways, including an XPS spectrum [1D, right] which is indebted to the carboxylated GO of [3].
Don't fear that the other panels of Figure 1 from [3] have been neglected. Panel 1(C) is postponed until [20]. As for 1(A), we find it illustrating an application of GO, in Figure 1 of paper [5] from Min Pan:
[5] The highly efficient adsorption of Pb(II) on graphene oxides: A process combined by batch experiments and modeling techniques (Xiaoming Huang & Min Pan, 2016).
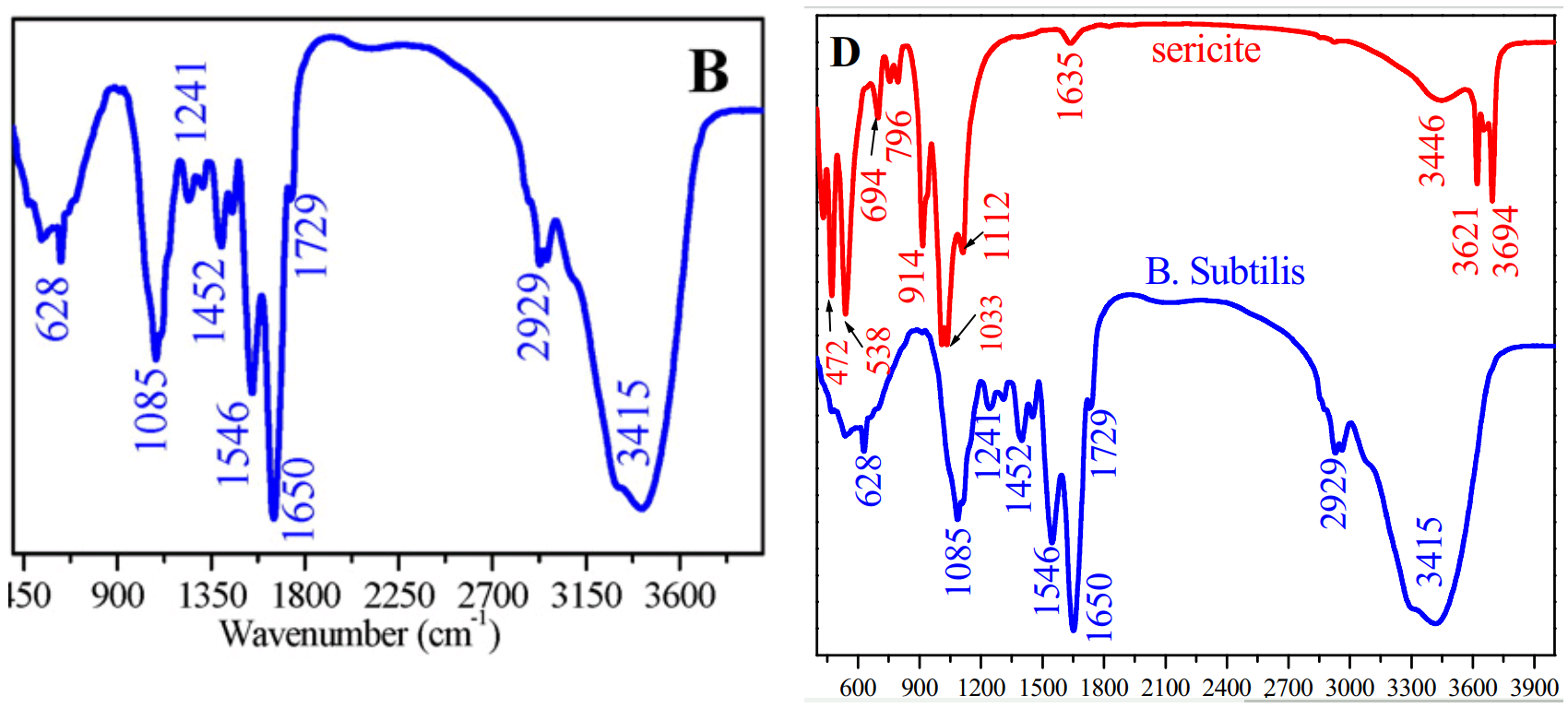
But we have not finished with Ting Yao's work. At left below is Figure 1 B, a FTIR spectrum characterising B subtilis, from
[6] Biosorption of Eu(III) and U(VI) on Bacillus subtilis: Macroscopic and modeling investigation (Yao, ...& Li, 2016).
It is shared at right, in common with the lower FTIR spectrum of Fig 1D in
[7] Adsorption of U(VI) on sericite in the presence of Bacillus subtilis: A combined batch, EXAFS and modeling techniques (Sun et al. 2016).
Now the final and corresponding author of [6] was Fengbo Li, a new arrival in the dramatis personae. His scholarly endeavours at Huangshan University have focused on applications of a soil fungus, Paecilomyces catenlannulatus - in particular, its potential for sequestering and bioabsorbing heavy metals. There is a recurring theme here, of dealing with environmental contaminants of the breathtakingly-toxic and sometimes radioactive variety, reflecting the downside of China's industrial transformation. Anyway, Li serves as a link to a third cluster of researchers with their own pair of papers.
[8] Adsorption of U(VI) on magnetic iron oxide/Paecilomyces catenlannulatus composites
(Fengbo Li, Xiaoyu Li & Pu Cui, 2018).
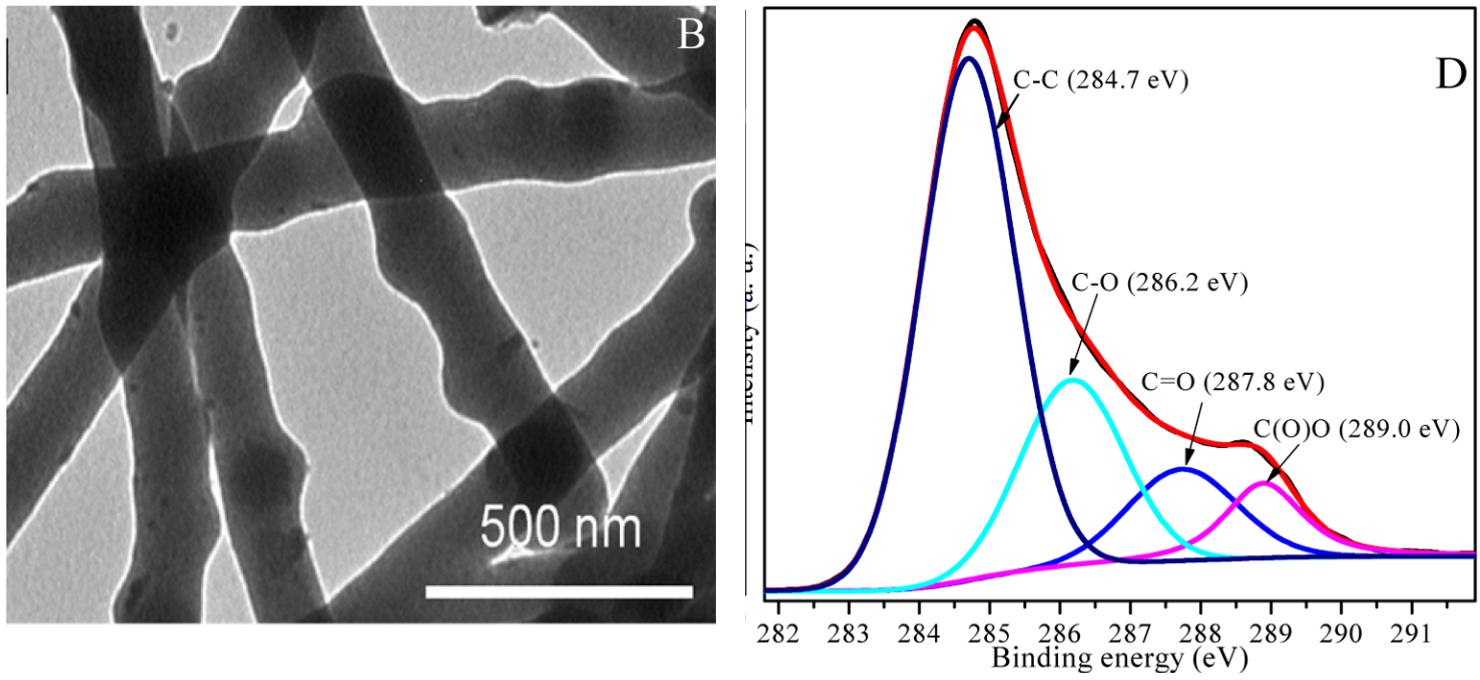
Figure 1 is what catches the eye, for the "MIO/B composites" shown there are reminiscent of the "carbon nanofibers" depicted in another Figure 1 from a different paper:
 Progressing quickly to a second paper from these authors, its Fig. 1(A) (a FT-IR spectrum for P. catenlannulatus) reveals an unusual repeated feature, rather resembling a front view of a sofa. Somehow it is turning into an airliner seating diagram.
Progressing quickly to a second paper from these authors, its Fig. 1(A) (a FT-IR spectrum for P. catenlannulatus) reveals an unusual repeated feature, rather resembling a front view of a sofa. Somehow it is turning into an airliner seating diagram.[10] Detoxification of U(VI) by Paecilomyces catenlannulatus investigated by batch, XANES and EXAFS techniques (Fengbo Li, Xiaoyu Li & Pu Cui, 2018).
The paper also provides a useful segue to EXAFS. To repeat, this is high-end materials science. I would expose the shallowness of my understanding if I tried to explain EXAFS. The crux, though is that at high enough resolution, the fine structure of an X-ray absorption line contains clues to the crystalline neighbourhood of the atoms absorbing those X-rays (because the probability that a photon will dislodge an electron is modulated by the energy-dependent interference between that dislodged electron, as a wave, and neighbouring atoms / bonds). A computation-heavy weighted Fourier transform from the frequency into the spatial domain makes these clues explicit.
The only source of sufficiently pure monochromatic X-rays is a synchrotron beam-line, and these are not desktop laboratory devices (though there is one in Shanghai). So not many people are active in the field. Evidently Fengbo Li qualified for access to the Shanghai installation (as well as access to radionuclides), resulting in Figure 6(B) [left], where the bottom profile is an EXAFS spectrum for "U-loaded P. catenlannulatus incubation time = 7 days, pH 4.5, CU(VI) = 20 mg/L."
Unexpectedly, all three spectra are replotted from counterparts in Fig. 7(B) [right], from
[11] Simultaneous adsorption and reduction of U(VI) on reduced graphene oxide-supported nanoscale zerovalent iron (Sun et al. 2014).
To sum up: we have three small clusters of researchers located around China, repurposing images and results from the large, productive Sun / Wang group. In comments in Pubpeer, Sun and Wang have expressed dismay at these acts of intellectual piracy, while preferring not to speculate about exactly how the other groups obtained the Ur-versions. Those clusters are not entirely disjunct: Fengbo Li of the third group was corresponding author on Yao's paper [6]. Li has also collaborated with Sun, most recently in 2018 (see [12] below).
 I should note that much of the pirated material was published in the Journal of Molecular Liquids, which benefits from Xiangke Wang's presence on its Editorial Board; it is a pity that papers [4], [5], [6], [8] did not cross his desk and alert him to these misdeeds.
I should note that much of the pirated material was published in the Journal of Molecular Liquids, which benefits from Xiangke Wang's presence on its Editorial Board; it is a pity that papers [4], [5], [6], [8] did not cross his desk and alert him to these misdeeds.Perhaps in compensation, there are some unusual patterns of citation (as promised above). In [4], 26 out of 44 references invoke the work of Y. B. Sun. In [5]. 15 out of 44. I am not convinced that they all crucially relevant.


Citations, of course, have become the currency of the academic-publishing economy. Like viewers' votes in a Reality-TV show, they drive the Impact Factors of journals, and of individual papers. Summed across papers, they define the importance and determine the career paths of the authors, removing the need for science administrators to understand anything of what people might have contributed before promoting them or bestowing tenure. Roles on Peer-Reviewer panels and Editorial Boards are determined by citations in all their majestic objectivity.
27 out of 51 references in [6] invoke Y. B Sun. In [8], 27 of 47. This might strike some as immoderate.


This creates an incentive for authors to pack self-citations into every niche of their own References sections, or as peer-reviewers, to render judgement that a manuscript is unpublishable until it contains 12 citations of one's own work; though these are generally deprecated. Citation cartels at the level of journals - mutually-beneficial exchanges of backrolling and logscratching between editors - are derogated in the same way.
In [10], 23 of 38 citations. In [12], 20 of 56 citations. Here [12] is the Li / Sun collaboration foreshadowed above:
[12] Plasma-grafted amidoxime/metal–organic framework composites for the selective sequestration of U(vi) (Fengbo Li, Xiaoyu Li, Pu Cui & Yubing Sun, 2018).


Now [12] commends itself to our attention partly for Figure 1(B), a FT-IR spectrum for an amidoxime/metal–organic framework composite or AO/MOF, containing odd segments of self-similarity.


Those features were already present when the spectrum was published a year earlier - vertically compressed - as the upper, red profile in Figure 1(C) of [13]:
[13] Plasma-Facilitated Synthesis of Amidoxime/Carbon Nanofiber Hybrids for Effective Enrichment of 238U(VI) and 241Am(III) (Sun et al. 2017).
Indeed, the two papers shared any number of results, despite the difference between AO/MOFs and "Amidoxime/Carbon Nanofiber Hybrids". Xiangke Wang was dismayed by the theft but we have yet to hear from Sun, the author in common.
EXAFS results were shared.
In fact these spectra make a third appearance! They are also Figure 5 [at left] of [14]
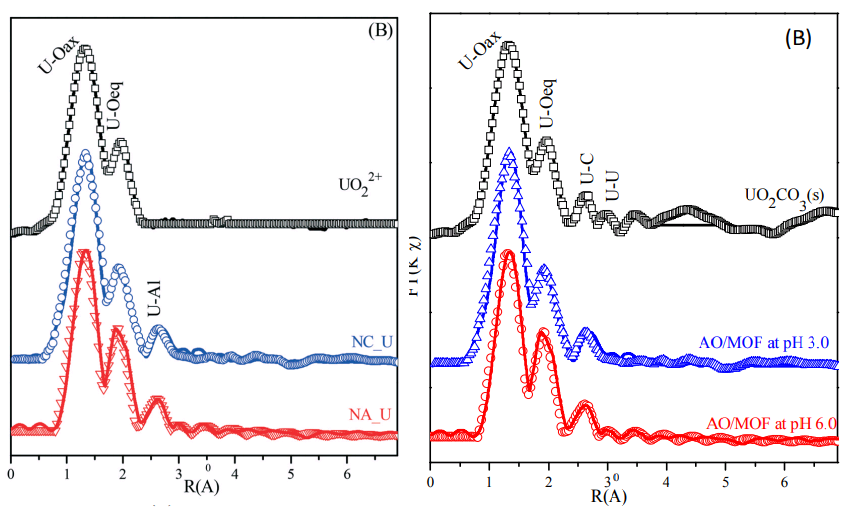
[14] The influence of nanoscale size on the adsorption–desorption of U(vi) on nano-Al oxides (Wan et al. 2018).
 In that third home, two of the spectra donned new identities as uranium-sequestering nano-alumina and nano-corundum. The uppermost spectrum (U(vi) in isolation) is unchanged for R(Å) < 2.2, but for R(Å) > 2.2 it is flat. The absence of a third peak piqued the curiosity of an EXAFS expert in a Pubpeer comment but the best explanation is that the output was edited.
In that third home, two of the spectra donned new identities as uranium-sequestering nano-alumina and nano-corundum. The uppermost spectrum (U(vi) in isolation) is unchanged for R(Å) < 2.2, but for R(Å) > 2.2 it is flat. The absence of a third peak piqued the curiosity of an EXAFS expert in a Pubpeer comment but the best explanation is that the output was edited.There is just time to admire two Brokenspectrum from [14] before continuing: To the right, some experimental hiccoughs have perturbed Figure 5(A), showing XPS analyses for the two aluminium oxides.
We are now within Sun's canon but there is no space to be exhaustive (readers are invited to explore the relevant PubPeer threads). I shall quickly select a few representative Brokenspectra.
[15] Determination of chemical affinity of graphene oxide nanosheets with radionuclides investigated by macroscopic, spectroscopic and modeling techniques (Ding et al, 2014).
[16] Enhanced adsorption of ionizable aromatic compounds on humic acid-coated carbonaceous adsorbents (Sun et al. 2012).
[17] Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline (Sun et al. 2013).
[18] Mechanistic insights into the decontamination of Th(iv) on graphene oxide-based composites by EXAFS and modeling techniques (Sun et al. 2017).


The upper one on the right is really the Prague skyline.
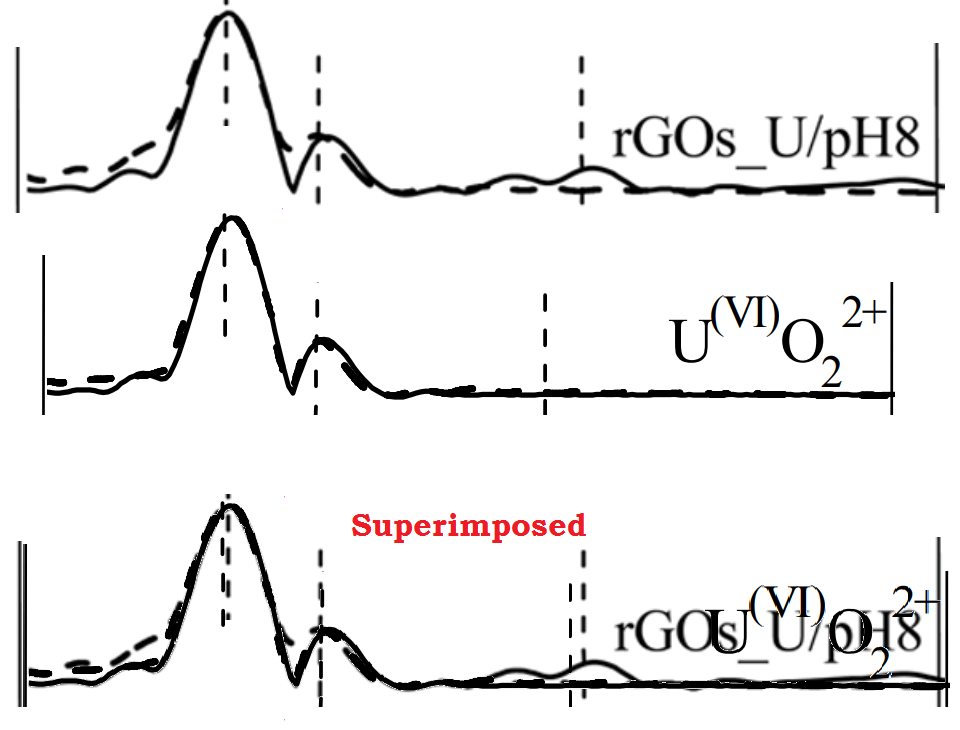
I really want to concentrate on the EXAFS analyses, for they are the point of difference for this group. So it was natural to take seven raw-data and transformed spectra for functionalized GO, in Figure 4 of [3] [below, left], and compare them against the transformed spectra [right] for "uranium-containing nZVI/C composites" at different pH / time combinations, from [19]. Mirabile dictu, four of them are the same!
[19] New Synthesis of nZVI/C Composites as an Efficient Adsorbent for the Uptake of U(VI) from Aqueous Solutions (Liu et al. 2017).
The similarity is not complete, for when the 2015 "rGOs_U/pH8" profile was repurposed to be "U(VI)O22+ in 2017, it was edited to be flat for R(Å) &rt; 2.5.
As always, there is more, and I am compelled us if by a gaes to take those seven spectra from [3] and compare them again... four of them also appeared as Fig. 4 in [2], this time representing sonochemically-tuned Uranium-sponge GO variants.
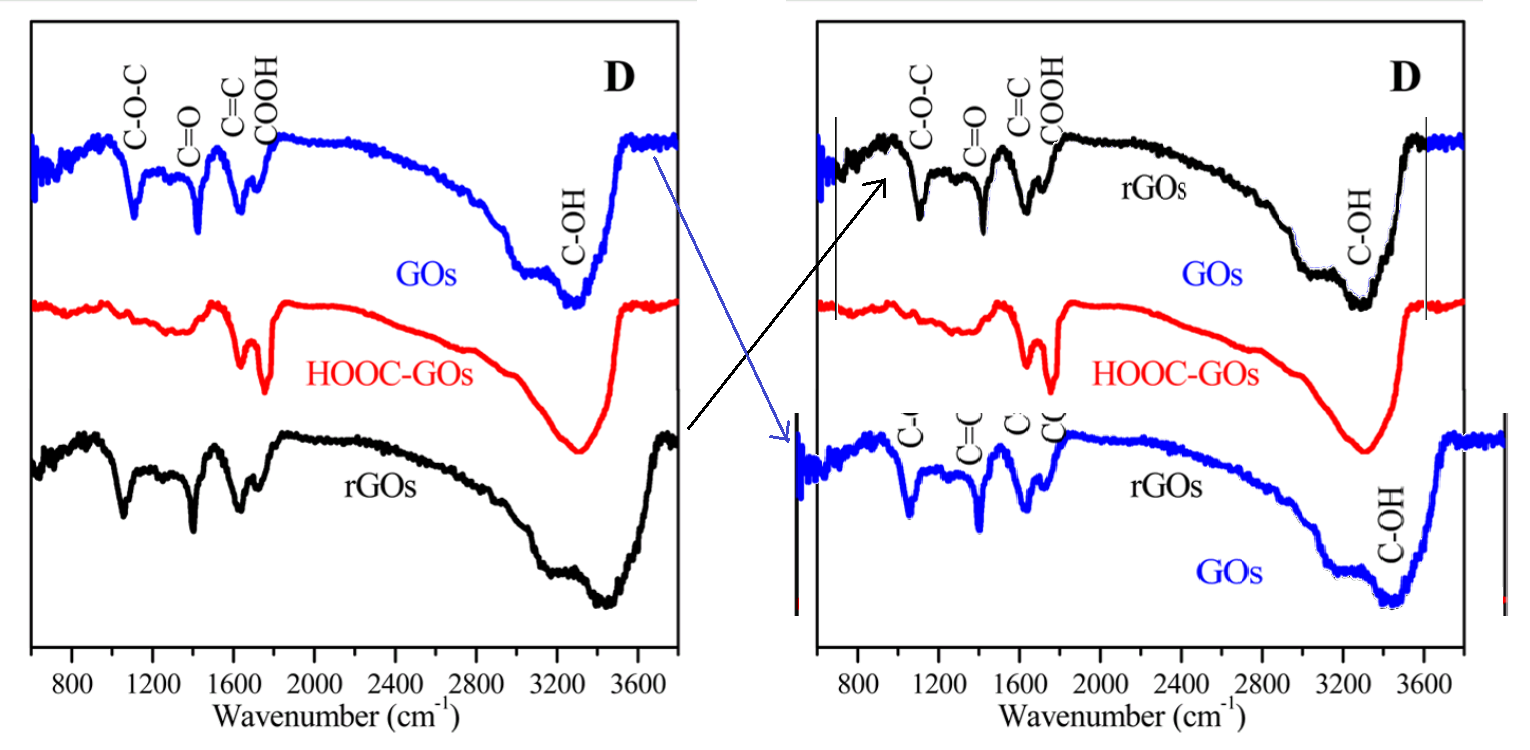
Now that we are back on the subject of [3], with the functionalized GOs, it may be time to wonder why the FT-IR spectra for GOs and rGOs are identical, down to pixels of noise, except for a 10% horizontal stretch.
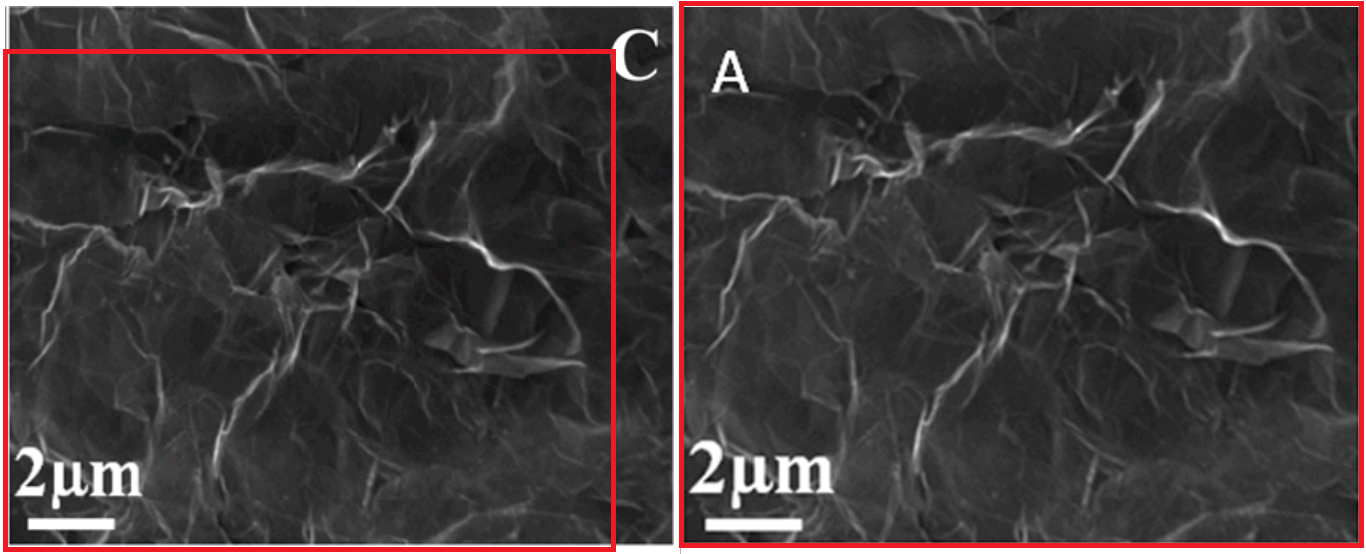
Readers might be worried about Panel 1(C) from [3] - a crumpled paperbag of a reduced-GO sheet, seen via SEM. Don't worry, it has not been neglected, and was transformed back into GO [here at right] to become Figure 1(A) of [20].
[20] High sorption of U(VI) on graphene oxides studied by batch experimental and theoretical calculations (Wang et al. 2016).
And going back to the Graphene Oxide SEM of [3] Fig 1(A), there are two other reported sightings. One was in Supplementary Figure S1A of [21], which again described the subject matter as GO, so the main concern is that a different microscope had reportedly created the image.
[21] Mutual effect of U(vi) and Sr(ii) on graphene oxides: evidence from EXAFS and theoretical calculations (Cheng et al., 2017),
The second was in Fig. 1D [right] of [22], where in the manner of a master-criminal from sensationalist fiction, the material had changed its identity, and was calling itself a "mesoporous Al2O3/EG composite".
[22] Enhanced adsorption of Eu(III) on mesoporous Al2O3/expanded graphite composites investigated by macroscopic and microscopic techniques (Sun et al. 2012)
* * * * * * * * * * * * * * * *
What is one to make of all this? Sun and Wang have reached the pinnacle of Physical Chemistry success, and ascended into the High-Citation empyrean (assisted by a certain degree of self-citation). When you have gained access to (1) exotic materials, and (2) a synchrotron X-ray beamline, with (3) software and (4) grad students to write the manuscripts, then a stream of publishable papers is guaranteed (along with the funding to ensure that (1)-(4) continue). There should be no need to manipulate spectra, and duplicate results, and extract different, incompatible spectra from single sets of data.In particular, there should be no need for groups of outsiders to this niche of materials science, embellishing their academic records with papers consisting of images from Sun & Wang, text in the style of Sun & Wang, and citations to Sun & Wang. One's papers (and the broader plot arcs they build) should already be attracting citations from other researchers in the field. Assuming, of course, that other researchers in the field can replicate one's results and calculations.
[Thanks TigerBB8 for contributions]
















No comments:
Post a Comment