This post was earlier cross-posted at Leonid Schneider's site, hence the unfrivolous tone. The version there is improved by Leonid's editing, background details and frame-story.
Does the world need another story about the European Review for Medical & Pharmacological Sciences as a conduit for papermills? The connection has already featured in an earlier post where the ERMPS was the journal most receptive to (and targeted by) "Papermill #2". It first came to our attention as the vehicle for a couple of papers by the talented Professor Miltonprabu, and most recently it was the star of a magisterial "Stock Photo Paper Mill" investigation by Elisabeth Bik.
But I am determined to give these publications the attention they deserve, and which they are not receiving now, for the ERMPS is a write-only journal, not designed to be accessed... it is not even equipped with Tables of Contents, and the NLM is not doing anyone any favours by indexing it in Medline and PubMed. Leonid Schneider will probably have opinions about the business model of the publisher, Verduci. ERMPS is not a journal that people follow to learn the latest clinical or laboratory milestones... the majority of its contents go unread, and any citations are to show the thoroughness of someone's literature search and the importance of whatever niche they have chosen to study. They turn the prayer-wheels of promotion and dissipate into oblivion from which I wish to rescue them here.
Also, I am constitutionally incapable of passing a dead horse without flogging it, so here we go! My lovingly-curated gallery of bogus papers is here, though only
One pair of papers reduces the papermill modus operandi to its essences. Liu et al (2017) [2] and Wang et al (2018) [4] were woven around the same Western Blots and bar-graphs, though replacing UCP1 with OPA, "liver injury caused by LPD" with "osteoporosis induced by radiation", and "EKR signaling pathway" with "P38 signaling pathway".


To be fair, the text is not a mere duplication; the anonymous papermill scribes have customised everything for the two separate contexts, They are veritable Renaissance polymaths, familiar with the nomenclature of multiple biomedical sub-fields, well enough to plausibly describe non-existent experiments in any of them. They vary the phrasing enough to make the textual cloning less obvious to G**gle searches.
"Papermills" here is a convenient rubric for the ateliers or foundries which forge spurious scientific results for publication, on an industrial scale... not from some malign desire to drown out or dilute the legitimate scientific results (which have a slightly higher chance of being correct), but to meet the career exigencies of the nominal authors who buy these fabrications and sign their names to them if and when they're accepted by a sufficiently amenable journal. Without the contributions of papermills, it's doubtful that Bioscience Reports could have become home to so many paper-shaped artifacts.
Each production is a collage of made-up answers to some minor biomedical research question, replete with Western Blots or flow-cytometry scatter-plots or immunohistochemistry-stained tissue slices or cell-proliferation microphotographs, guided by the rigid conventions of the genre. These convincing circumstantial illustrations might be filched from other authors; or composed entirely in the digital domain of Photoshop; or actually sourced from experiments, for some papermills seem to be side-projects of legitimate research institutes making a little extra cash on the side. Often they are recycled across papers, as if the archives of imagery are limited and the papermills cannot afford to be profligate with them (though it may be different for the mills we don't know about). So here are some closing clam-shells...
...No wait, I tell a lie, they are echocardiograms from rats, repurposed to show the effect of Sitagliptin (Wu et al 2019 [6]) and miR-548c-3p (Zhang et al 2019 [13]) on heart function after surgical simulation of a rodential heart attack. The papermill evidently can tap into a supply of Ischaemia/Reperfusion-method echocardiograms, even if they sometimes reuse them out of carelessness or necessity.
Here, the same WB loading controls in two unrelated papers by unconnected research teams: Yang et al (2017) [1] and Lu et al (2018) [3].
The productions might be composed 'on spec' (by systematically permuting the themes and working through possible combinations) and put on show like puppies in a pet-shop window for prospective authors to bond with, or bespoke - tailored to fit a customer's specific research grant and supposed area of expertise. Sometimes would-be authors form ad-hoc coalitions to share the costs.
An equivalent in the mainstream literary world might be the "romance novel" genre of fiction with its equally convention-bound structure. One might also be reminded of The Silver Eggheads (1962), in which Fritz Leiber imagined computerised Wordmills filling the need for disposable word-wooze and taking over from human authors (until saboteurs destroy the wordmills and bring about a fictional famine). Nevertheless, I prefer to imagine the ateliers as something like a mediaeval monastic scriptorium, where tonsured monks toil over manuscripts by candlelight, perhaps with a division of labour between the limners composing the Figures and the scribal copyists filling in the gaps between these with suitable Methods sections and assembling the appropriate Introductions.
My search of the ERMPS back-catalog focused, to begin with, on a tradition of 'Sham / Model / treatment' illustrations, comparable to a Bosch altarpiece. Each left-hand panel, 'Earthly Paradise', presents a prelapsarian scene of heart tissue (or brain, or kidneys) after a sham Control operation inflicting no actual damage; the central panel is a hellish scene of cellular devastation wrought in imitation of some disease or condition; while the right-hand panel, 'Redemption', depicts the restorative effect of the treatment of choice. It might even be a manipulated version of the left-hand image, or all three could be digital constructs that wandered in from Virtual Reality. More specifically, I began with this trio in Figure 2 of Zhang et al (2019) [20], where the myocardial fibres at left were flipped through 180° and subjected to some desultory retouching to produce the version on the right.


So in my mind this is a Triptych Papermill, though diptychs and more complex polyptych altarpieces are possible. Figure 2 of Bian et al (2019) [8] contained only two frames, while Fig 1 of Bai et al (2019) [22] was back to three.


But that's not all, for a later paper by Bai et al (2020) [25] leads back to [20], through the presence of similar mildly-manipulated constellations of TUNEL apoptoses, Figs 2A and 3A respectively:


While Fig 2 of [8] links further afield to Fig 3 of Pan et al (2019) [16], with Bax and Bcl-2 stains swapping their masks in the process:


Of course these observations are only possible because the papermill concentrates on one journal as a pipeline for their pictorial repurposing. The cooperation between ERMPS and papermills is convenient for them, but also for us: other versions of the same recycled image are likely to go unnoticed when they fly off to other journals elsewhere in the scholarly galaxy (such as Experimental and Therapeutic Medicine from the Spandidos stable).
I was quickly distracted by this reuse of images between papermill creations, and my attention broadened to stylistic departures from the Triptych paradigm, so I cannot guarantee that my regrettable gallery all came from the same studio. We already know that multiple mills are taking advantage of the ERMPS complaisance (or that ERMPS is taking advantage of payments from multiple mills).
To stick with the myocardial theme, I have doubts about the veridicality of these images from Zhang et al (2019) [21], though no doubt considerable effort went into their construction..
Four more triptychs overlap, both internally: Figs 3 of Zhou et al (2019) [11] and 2A of Xu, Xu & Wang (2019) [17]...


...and between those unrelated papers. Figs 2 of [11] and 1 from [17].
Another example, because why not? Fig 3 from Wang et al (2019) [19], meet Fig 5 from Xie et al (2020) [24]. Fig 7 from [24], meet Fig 6 from [19]. But perhaps you already know one another.


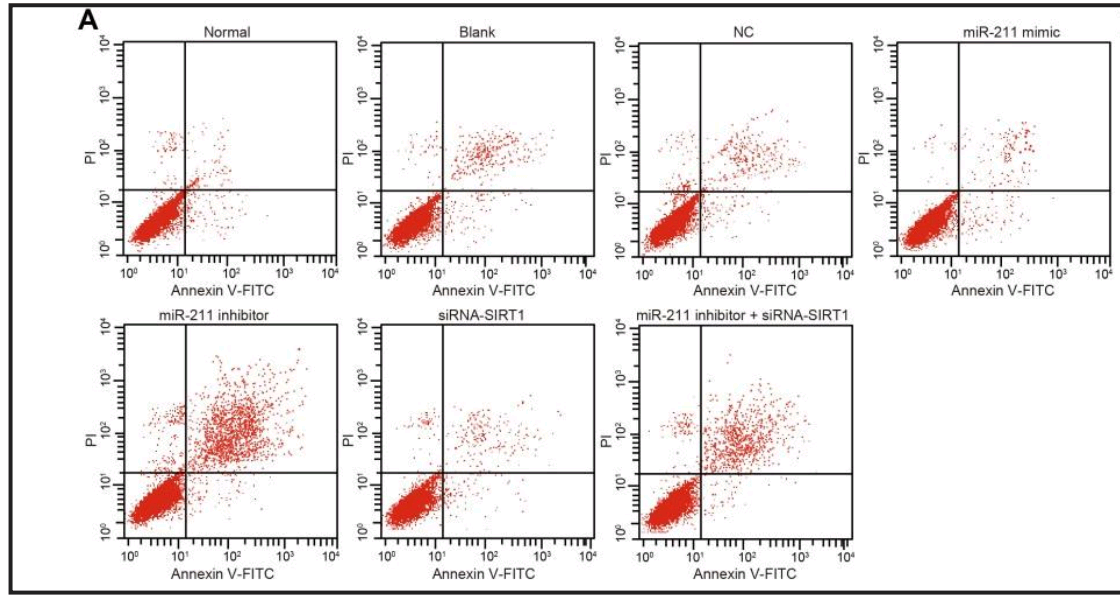
For variety, some Flaw Cytometry. Does miR-146 mediate the NF-κB signaling pathway to protect diabetic nephropathy from kidney injury, as in Yu et al (2020) [30]?
Unless what we see is miR-210, regulating the VEGF-notch signaling pathway to suppress neuronal apoptosis in rats with cerebral infarction, as in Jiang et al (2020) [31]... or lncRNA MEG3, regulating PTEN to inhibits proliferation and promotes apoptosis of synovial cells in rats with knee osteoarthritis, as in Han et al (2020) [32].


UPDATE: these flaw-cytometry plots first appeared in a paper in Cellular Physiology & Biochemistry, Zhang et al (2018). I'm not sure whether that one came from the papermill: it could have been an unwitting source of raw material.
It would be helpful to learn how a US doctor came to sign one of these productions. So far Dr Chayakrit Krittanawong has not responded to queries through social media or to the automagical email from PubPeer inviting him to join the discussion of the paper he purportedly co-authored, Sun et al (2019) [23]. In his defence, the medical staff at US hospitals possibly have pandemic-related activities to occupy their time, more urgent than questions of publication provenance.
Anyway, on the face of it, [23] appears to have copied images from Yin et al (2018) [18]:
The textual overlap is extensive, especially in the Introduction but interrupted in the Conclusions by the two papers' different diagnostic preoccupations.


[Thanks Tiger]
I prefer the more charitable interpretation, though, in which this is a more benign case of duplicate publication -- that is, both are the work of Anonymous, who wove the same fabricated or pirated images and the same made-up results into two manuscripts. One was sold to Yin et al., and the other to one of Krittanawong's colleagues, who in turn offered Krittanawong an honorary co-authorship. Being an expert on the etiquette of academic authorship (on account of his prestigious editorial appointments), Krittanawong decided that he could ethically accept.The highlight of my collection is a nexus of 14 papers, which together illustrate the entire range of medical misadventure that one might inflict on rats. We have already met [16], a gateway into the nexus. Other members, in order of publication are: Long et al (2019) [5]; Chang et al (2019) [7]; Zhao et al (2019) [9]; Sun et al (2019) [10]; Jiang et al (2019) [12]; Chen et al (2019) [14]; Lou et al (2019) [15]; Rong et al (2020) [26]; Cai et al (2020) [27]; Wang et al (2020) [28]; Lu & Lu (2020) [29]; Xie et al (2020) [33]; and Qi et al (2020) [34].
Some of them are interlinked by a trail of microphotographs in which myocardial and retinal tissues are interchangeable. Figure 1 from [14]:
Progressing from Bcl-2 to TUNEL-stains, Figures [6] from [14] and [29]:


The latter lends its 'Sham' panel to become the Control in Fig 6 of [33], in which Sham and Inhibitor panels are the same. Nor should we forget Fig 7 of [34] in which these images are showcased again.


What really ties this nexus together, though, is a small but versatile repertoire of WBs, with a couple of hard-working beta-actin loading controls. Upper right here, Figure 3 from [27]. Upper, middle and lower left: Figs 3 from [29], [14], and [33].
There was no room in that chart for Fig 3 from [7] and Fig 4 from [28].


But the substantive bands in this repertoire are equally versatile (though inconstant in their affiliations), and those last two figures have more in common when 180° rotations are considered: see below at left. Below at right, applying the same perspective to Figs 4 and 3 from [26] and [29].


[26] can boast of a different row of loaves as a loading control, shared only with [22] - another gateway to the nexus!
The left-hand combination, below, shows (clockwise from upper left): Figure 3 from [10]; Fig 3 from [5]; Fig 4 from [15]; and Figure 5 from [16]. But that diagram left out (at right) Fig 3 of [9].


On the off-chance that the dead horse is not yet sufficiently flogged, two last diagrams. This combination consists of Figs 3 from (clockwise from upper left) [27], [12] and [34] (stretched to 120% vertically for convenience). Then, from upper to lower left: Figure 4 from [15], Fig 3 from [14], and Fig 3 from [33].
ERMPS was founded before the modern development of an ecosystem of predatory publishing, so it has not always been simply a pipeline for papermills. The website offers a print-on-demand Hardcopy option for an additional fee. From this I infer that it was once more of a vanity publisher, targeting authors who conducted actual study and wrote their own papers, and took enough pride in them to adorn their offices with physical copies.
Here's the link to the spreadsheet again. It in turn contains links to the corresponding PubPeer threads, and to the papers themselves. To give readers an incentive to explore those threads, here are two final polyptychs, but I won't say which papers they came from.
Sources:
[1]. “Regulation of miR-33b on endometriosis and expression of related factors“, W-W Yang , L Hong, X-X Xu, Q Wang, J-L Huang, L Jiang (2017). DOI: 28537685 [PubPeer][2]. “Mitochondrial protein UCP1 mediates liver injury induced by LPS through EKR signaling pathway“, P Liu , J Yang, Z-Y Chen, P Zhang, G-J Shi (2017). DOI: 28925474 [PubPeer]
[3]. “MiR-155 affects osteosarcoma cell proliferation and invasion through regulating NF-κB signaling pathway“, S. Lu, Q.-S. Liao, L. Tang (2018). DOI: 10.26355/eurrev_201811_16380 [PubPeer]
[4]. “Mitochondrial protein OPA mediates osteoporosis induced by radiation through the P38 signaling pathway“, W.-D. Wang , W.-B. Kang, X.-Q. Zhou, G.-F. Yao, X.-J. Wang (2018). DOI: 10.26355/eurrev_201812_16499 [PubPeer]
[5]. “Research on the expression of MRNA-518b in the pathogenesis of placenta accreta“, Y. Long , Y. Chen, X.-Q. Fu, F. Yang, Z.-W. Chen, G.-L. Mo, D.-Y. Lao, M.-J. Li (2019). DOI: 10.26355/eurrev_201901_16743 [PubPeer]
[6]. “Sitagliptin inhibits EndMT in vitro and improves cardiac function of diabetic rats through the SDF-1α/PKA pathway“, Y. Wu , M. Xu, H. Bao, J.-H. Zhang (2019). DOI: 10.26355/eurrev_201901_16899 [PubPeer]
[7]. “Influence of roflumilast on sepsis mice through the JAK/STAT signaling pathway“, X. Chang, L.-F. Hu, X.-J. Ma, J. Yin, X.-Y. Liu, J.-B. Li (2019). DOI: 10.26355/eurrev_201902_17028 [PubPeer]
[8]. “Influence of miR-34a on myocardial apoptosis in rats with acute myocardial infarction through the ERK1/2 pathway“, W.-S. Bian , F.-H. Tian, L.-H. Jiang, Y.-F. Sun, S.-X. Wu, B.-F. Gao, Z.-X. Kang, Z. Zhuo, X.-Z. Zhang (2019). DOI: 10.26355/eurrev_201904_17585 [PubPeer]
[9]. “MiR-29 regulates retinopathy in diabetic mice via the AMPK signaling pathway“, B.-W. Zhao , H.-Y. Dai, L.-N. Hao, Y.-W. Liu (2019). DOI: 10.26355/eurrev_201905_17778 [PubPeer]
[10]. “Expression of NF-κB in juvenile rats with nephrotic syndrome and its effects on inflammatory changes and renal injury“, X.-M. Sun, X.-H. Sun, Z.-Y. Li, C.-J. Yuan, J. Wu (2019). DOI: 10.26355/eurrev_201905_17831 [PubPeer]
[11]. “MiR-101a attenuates myocardial cell apoptosis in rats with acute myocardial infarction via targeting TGF-β/JNK signaling pathway“, F.-Q. Zhou , X.-F. Zhao, F.-Y. Liu, S.-S. Wang, H.-L. Hu, Y. Fang (2019). DOI: 10.26355/eurrev_201905_17952 [PubPeer]
[12]. “A study on regulatory mechanism of miR-223 in ulcerative colitis through PI3K/Akt-mTOR signaling pathway“, W. Jiang, Y.-P. Han, M. Hu, X.-Q. Bao, Y. Yan, G. Chen (2019). DOI: 10.26355/eurrev_201906_18074 [PubPeer]
[13]. “Role and mechanism of microRNA-548c-3p/c-Myb in myocardial infarction fibrosis in rats“, L.-X. Zhang , S.-H. Zhang, C.-Q. Wang, Q. Bing, Z. Zhao, J. Wang, L. Zhang (2019). DOI: 10.26355/eurrev_201906_18081 [PubPeer]
[14]. “Effect of propofol on myocardial ischemia/reperfusion injury in rats through JAK/STAT signaling pathway“, X. Chen , Y. Wang, Z.-Y. Xiao, D.-N. Hou, D.-B. Li, X.-P. Zhang (2019). DOI: 10.26355/eurrev_201907_18456 [PubPeer]
[15]. “Role of miR-21 in rats with proliferative diabetic retinopathy via TGF-β signaling pathway“, H.-D. Lou, S.-Y. Wang, T. Guo, Y. Yang (2019). DOI: 10.26355/eurrev_201908_18621 [PubPeer]
[16]. “Promethazine inhibits neuronal apoptosis via PI3K/Akt signaling pathway in rats with cerebral infarction“, X.-D. Pan , X.-L. Chen, S.-F. Ding, D. Kou, H.-L. Hu, L. Li (2019). DOI: 10.26355/eurrev_201908_18639 [PubPeer]
[17]. “Effect of exosome-carried miR-30a on myocardial apoptosis in myocardial ischemia-reperfusion injury rats through regulating autophagy“, Y.-Q. Xu , Y. Xu, S.-H. Wang (2019). DOI: 10.26355/eurrev_201908_18748 [PubPeer]
[18]. “Knocking down PFL can improve myocardial ischemia/reperfusion injury in rats by up-regulating heat shock protein-20“, R.-L. Yin , H. You, Y.-M. Wu, F.-L. Ye, W.-X. Gu, J. Shen (2019). DOI: 10.26355/eurrev_201909_18885 [PubPeer]
[19]. “Influence of miR-34a on cerebral neuronal apoptosis in rats with cerebral ischemia reperfusion through the Notch1 signaling pathway“, S.-P. Wang , D. Wang, H.-X. Li, L. Liu, X.-H. Duan (2019). DOI: 10.26355/eurrev_201909_19021 [PubPeer]
[20]. “Silence of lncRNA XIST represses myocardial cell apoptosis in rats with acute myocardial infarction through regulating miR-449“, M. Zhang , H.-Y. Liu, Y.-L. Han, L. Wang, D.-D. Zhai, T. Ma, M.-J. Zhang, C.-Z. Liang, Y. Shen (2019). DOI: 10.26355/eurrev_201910_19172 [PubPeer]
[21]. “Effect of lncRNA GAS5 on rats with acute myocardial infarction through regulating miR-21“, J.-C. Zhang , L. Xia, Y. Jiang, D.-Q. Wu, S.-C. Liu, X.-N. Zhou, F.-X. Zhang (2019). DOI: 10.26355/eurrev_201910_19173 [PubPeer]
[22]. “Effects of butylphthalide on oxidative stress and inflammatory response in rats with myocardial infarction through Akt/Nrf2 signaling pathway“, M. Bai, C.-L. Pan, G.-X. Jiang, Y.-M. Zhang, Z. Zhang (2019). DOI: 10.26355/eurrev_201911_19458 [PubPeer]
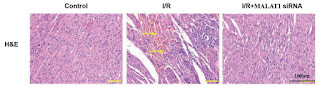
[23]. “LncRNA MALAT1 knockdown alleviates myocardial apoptosis in rats with myocardial ischemia-reperfusion through activating PI3K/AKT signaling pathway“, T. Sun , Y.-T. Cheng, L.-X. Yan, C. Krittanawong, W. Qian, H.-J. Zhang (2019). DOI: 10.26355/eurrev_201912_19693 [PubPeer]
[24]. “Relationship between NogoA/NgR1/RhoA signaling pathway and the apoptosis of cerebral neurons after cerebral infarction in rats“, Y.-X. Xie , M. Zhang, C.-R. Zhang, F. Chen (2020). DOI: 10.26355/eurrev_202001_19924 [PubPeer]
[25]. “CircRNA 010567 improves myocardial infarction rats through inhibiting TGF-β1“, M. Bai, C.-L. Pan, G.-X. Jiang, Y.-M. Zhang (2020). DOI: 10.26355/eurrev_202001_19935 [PubPeer]
[26]. “MiR-29 inhibits neuronal apoptosis in rats with cerebral infarction through regulating Akt signaling pathway“, W Rong , L Yang, C-Y Li, X-T Wu, Z-D Zhou, W-L Zhu, Y Yan (2020). DOI: 10.26355/eurrev_202001_20068 [PubPeer]
[27]. “MiR-137 affects bone mineral density in osteoporosis rats through regulating RUNX2“, W.-L. Cai , W. Zeng, B.-Y. Zhu, H.-H. Liu, J.-L. Liu (2020). DOI: 10.26355/eurrev_202002_20152 [PubPeer]
[28]. “Effect of lncRNA AK125437 on postmenopausal osteoporosis rats via MAPK pathway“, H. Wang , Y.-K. Li, M. Cui, L.-H. Liu, L.-M. Zhao, X.-M. Wang (2020). DOI: 10.26355/eurrev_202003_20482 [PubPeer]
[29]. “MiR-26a inhibits myocardial cell apoptosis in rats with acute myocardial infarction through GSK-3β pathway“, S. Lu, Y. Lu (2020). DOI: 10.26355/eurrev_202003_20535 [PubPeer]
[30]. “Protective effect of miR-146 against kidney injury in diabetic nephropathy rats through mediating the NF-κB signaling pathway“, H.-Y. Yu , L.-F. Meng, X.-H. Lu, L.-H. Liu, X. Ci, Z. Zhuo (2020). DOI: 10.26355/eurrev_202003_20688 [PubPeer]
[31]. “MiR-210 suppresses neuronal apoptosis in rats with cerebral infarction through regulating VEGF-notch signaling pathway“, Y.-L. Jiang , W.-W. Liu, Y. Wang, W.-Y. Yang (2020). DOI: 10.26355/eurrev_202005_21188 [PubPeer]
[32]. “LncRNA MEG3 inhibits proliferation and promotes apoptosis of synovial cells in rats with knee osteoarthritis by regulating PTEN“, K. Han , F.-R. Wang, M.-Q. Yu, B. Xu (2020). DOI: 10.26355/eurrev_202005_21306 [PubPeer]
[33]. “Oxycodone inhibits myocardial cell apoptosis after myocardial ischemia-reperfusion injury in rats via RhoA/ROCK1 signaling pathway“, Y. Xie , C.-L. Ge, Z.-Y. Zhang, G.-X. Fei (2020). DOI: 10.26355/eurrev_202006_21535 [PubPeer]
[34]. “MiR-204 inhibits inflammation and cell apoptosis in retinopathy rats with diabetic retinopathy by regulating Bcl-2 and SIRT1 expressions“, F. Qi, X. Jiang, T. Tong, H. Chang, R.-X. Li (2020). DOI: 10.26355/eurrev_202006_21631 [PubPeer]

















1 comment:
Perusing Dr. Chayakrit Krittanawong's LinkedIn profile, I discovered a reference to a paper whose interrogatory title commanded my respect: "Is a combination of artificial intelligence and blockchain a perfect match ?" (my answer is, no, not yet; have you considered supplementing the combination with nanoparticles?), followed by a series of hashtags ending with #digitaltransformation. Well.
Post a Comment